We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1068
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
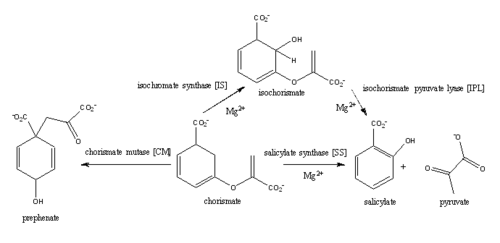
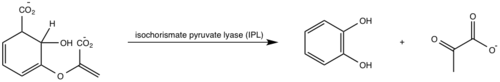
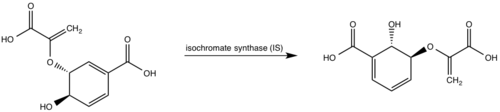
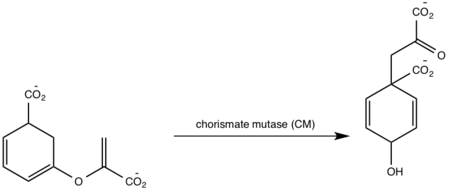
| - | <scene name='69/694235/3log/ | + | <scene name='69/694235/3log/10'>Salicylate synthase</scene> from ''Mycobacterium tuberculosis'' (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': [http://en.wikipedia.org/wiki/Isochorismate_synthase isochorismate synthase] (IS), [http://www.proteopedia.org/wiki/index.php/Isochorismate_pyruvate_lyase isochorismate pyruvate lyase] (IPL), [http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=8A8773E9&tabtoshow=Current salicylate synthase] (SS) and [http://en.wikipedia.org/wiki/Chorismate_mutase chorismate mutate] (CM). MtbI belongs to the chorismate-utilising enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/2'>Ipr9</scene>, <scene name='69/694235/Menf/2'>MenF</scene>, <scene name='69/694235/Entc/2'>EntC</scene>, and <scene name='69/694235/Mbti/2'>MbtI</scene>) that isomerize chromate to isochorismate. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation. IS, IPL, and SS activity are also modulated by the pH of the medium. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8. |
The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin, in ''Mycobacterium tuberculosis'' (Figure 3)<ref name= "5a">PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action <ref name= "1a"> DOI: 10.1002/cmdc/201000137</ref> <ref name= "2a">PMID:23108268</ref> <ref name= "7a">Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.</ref> <ref name= "5a"/> <ref name= "4a">DOI:10.1021/bi2009739</ref> <ref name= "9a">PMID:14982443</ref> | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin, in ''Mycobacterium tuberculosis'' (Figure 3)<ref name= "5a">PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action <ref name= "1a"> DOI: 10.1002/cmdc/201000137</ref> <ref name= "2a">PMID:23108268</ref> <ref name= "7a">Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.</ref> <ref name= "5a"/> <ref name= "4a">DOI:10.1021/bi2009739</ref> <ref name= "9a">PMID:14982443</ref> | ||
Revision as of 20:26, 25 April 2015
Contents |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1002/cmdc/201000137
- ↑ 3.0 3.1 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.
- ↑ Lamb AL. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug, 12. PMID:21823653 doi:http://dx.doi.org/10.1021/bi2009739
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ 8.0 8.1 8.2 Nicoloff H, Arsene-Ploetze F, Malandain C, Kleerebezem M, Bringel F. Two arginine repressors regulate arginine biosynthesis in Lactobacillus plantarum. J Bacteriol. 2004 Sep;186(18):6059-69. PMID:15342575 doi:http://dx.doi.org/10.1128/JB.186.18.6059-6069.2004
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
Student contributors
Stephanie Raynor Robin Gagnon