Sandbox Reserved 1068
From Proteopedia
| Line 11: | Line 11: | ||
==Structure== | ==Structure== | ||
| - | [[Image: | + | [[Image:Capture.PNG|300 px|left|thumb|'''Figure 2''': Monomeric ribbon diagram of MbtI with active site cleft highlighted with a white circle. Generated from [[3log]] (3a)]] |
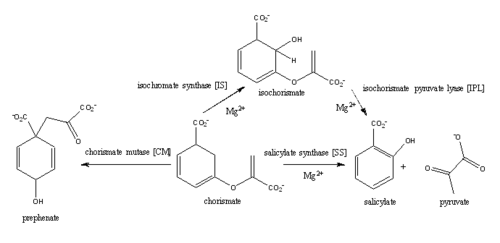
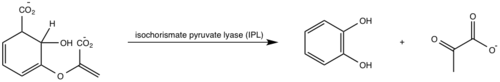
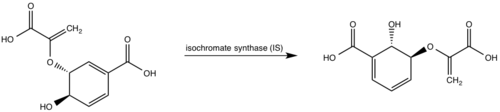
The crystal asymmetric unit was found to contain <scene name='69/694235/3log/11'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme <ref name= "3a">PMID 15342575</ref>. There are no significant structural changes between the four monomers excepts from the localized differences in the active site <ref name= "3a">PMID 15342575</ref>. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes <ref name="3a"/>. The core of the protein is formed by <scene name='69/694234/Beta_sheets/1'>21 Beta sheets </scene>folded into a twisted beta-sandwich. The protein's core is then surrounded by <scene name='69/694235/Beta_sheets/4'>10 alpha helices</scene><ref name="3a"/>. The active site was identified by comparison to the product bound forms of Irp9 and TrpE and is situated in a cleft that is about 12Å in length, 10Å deep, and 7Å wide. One side of the groove is formed by β21, C-terminal helix, and α11. The other side of the groove is formed by β16-17 loop, helix α7, and β15-α6 loop. The β19-20 and β12-13 loops make up the bottom of the active side cleft (Figure 2)(Harrison 2006) | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/11'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme <ref name= "3a">PMID 15342575</ref>. There are no significant structural changes between the four monomers excepts from the localized differences in the active site <ref name= "3a">PMID 15342575</ref>. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes <ref name="3a"/>. The core of the protein is formed by <scene name='69/694234/Beta_sheets/1'>21 Beta sheets </scene>folded into a twisted beta-sandwich. The protein's core is then surrounded by <scene name='69/694235/Beta_sheets/4'>10 alpha helices</scene><ref name="3a"/>. The active site was identified by comparison to the product bound forms of Irp9 and TrpE and is situated in a cleft that is about 12Å in length, 10Å deep, and 7Å wide. One side of the groove is formed by β21, C-terminal helix, and α11. The other side of the groove is formed by β16-17 loop, helix α7, and β15-α6 loop. The β19-20 and β12-13 loops make up the bottom of the active side cleft (Figure 2)(Harrison 2006) | ||
Revision as of 21:15, 26 April 2015
Contents |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1002/cmdc/201000137
- ↑ 3.0 3.1 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.
- ↑ Lamb AL. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug, 12. PMID:21823653 doi:http://dx.doi.org/10.1021/bi2009739
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ 8.0 8.1 8.2 8.3 Nicoloff H, Arsene-Ploetze F, Malandain C, Kleerebezem M, Bringel F. Two arginine repressors regulate arginine biosynthesis in Lactobacillus plantarum. J Bacteriol. 2004 Sep;186(18):6059-69. PMID:15342575 doi:http://dx.doi.org/10.1128/JB.186.18.6059-6069.2004
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
Student contributors
Stephanie Raynor and Robin Gagnon
Related pdb files and proteopedia pages
3D structures of isochorismate pyruvate lyase
3log – MtIPL/isochorismate synthase - Mycobacterium tuberculosis
3rv6, 3rv7, 3rv8, 3rv9, 3st6, 3veh - MtIPL/isochorismate synthase + inhibitor
2h9c – PaIPL residues 1-99 – Pseudomonas aeruginosa
2h9d - PaIPL + pyruvate
3LOG
3D structure of isochorismate synthase
2eua, 3bzm, 3bzn - MenF from E. coli
3os6 - DhbC from Bacillus anthracis
3gse - MenF from Yersinia pestis
3hwo - EntC
3D structure of salicylate synthase
3veh - MbtI with inhibitor methylAMT
3st6 - MbtI with isochorismate analogue inhibitor
3rv6 (Phenyl R-group), 3rv7 (Isopropyl R-group), 3rv8 (Cyclopropyl R-group), 3rv9 (Ethyl R-group) - MbtI with inhibitor
2fn0, 2fn1 (with products salicylate and pyruvate) - Irp9 from Yersinia enterocolitica
2i6y - MbtI