Sandbox Reserved 1068
From Proteopedia
| Line 5: | Line 5: | ||
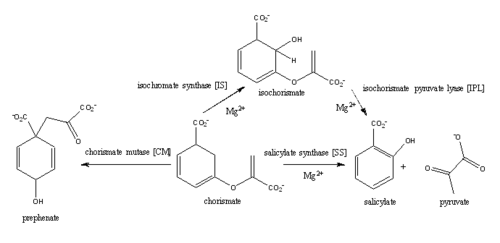
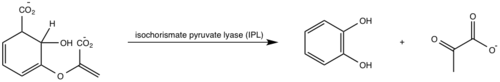
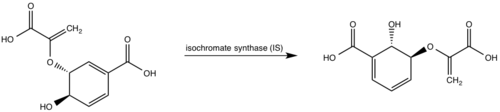
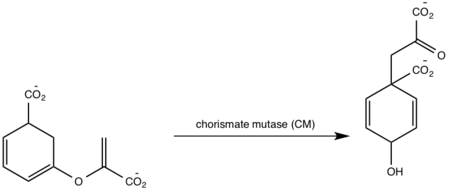
<scene name='69/694235/3log/12'>Salicylate synthase</scene> from [http://en.wikipedia.org/wiki/''Mycobacterium_tuberculosis''] (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': [http://en.wikipedia.org/wiki/Isochorismate_synthase isochorismate synthase] (IS), [http://www.proteopedia.org/wiki/index.php/Isochorismate_pyruvate_lyase isochorismate pyruvate lyase] (IPL), [http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=8A8773E9&tabtoshow=Current salicylate synthase] (SS) and [http://en.wikipedia.org/wiki/Chorismate_mutase chorismate mutate] (CM)<ref name="8a">PMID:22307014</ref>. MtbI belongs to the chorismate-utilizing enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/5'>Ipr9</scene>, <scene name='69/694235/Menf/3'>MenF</scene>, <scene name='69/694235/Entc/3'>EntC</scene>, and <scene name='69/694235/Mbti/3'>MbtI</scene>) that isomerize chromate to isochorismate and share a fold of two α/β subdomains, each comprising of a antiparallel β-sheet with helices packed against it <ref name="8a">PMID:22307014</ref> <ref name="1a">PMID:20512795</ref>. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis <ref name="4a">PMID:21823653</ref> <ref name="1a">PMID:20512795</ref> <ref name="7a">PMID:10655517</ref>. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation <ref name="8a">PMID:22307014</ref>. IS, IPL, and SS activity are also modulated by the pH of the medium <ref name="8a"/>. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8 <ref name="8a"/> <ref name="6a">PMID:17240979</ref>. | <scene name='69/694235/3log/12'>Salicylate synthase</scene> from [http://en.wikipedia.org/wiki/''Mycobacterium_tuberculosis''] (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': [http://en.wikipedia.org/wiki/Isochorismate_synthase isochorismate synthase] (IS), [http://www.proteopedia.org/wiki/index.php/Isochorismate_pyruvate_lyase isochorismate pyruvate lyase] (IPL), [http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=8A8773E9&tabtoshow=Current salicylate synthase] (SS) and [http://en.wikipedia.org/wiki/Chorismate_mutase chorismate mutate] (CM)<ref name="8a">PMID:22307014</ref>. MtbI belongs to the chorismate-utilizing enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/5'>Ipr9</scene>, <scene name='69/694235/Menf/3'>MenF</scene>, <scene name='69/694235/Entc/3'>EntC</scene>, and <scene name='69/694235/Mbti/3'>MbtI</scene>) that isomerize chromate to isochorismate and share a fold of two α/β subdomains, each comprising of a antiparallel β-sheet with helices packed against it <ref name="8a">PMID:22307014</ref> <ref name="1a">PMID:20512795</ref>. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis <ref name="4a">PMID:21823653</ref> <ref name="1a">PMID:20512795</ref> <ref name="7a">PMID:10655517</ref>. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation <ref name="8a">PMID:22307014</ref>. IS, IPL, and SS activity are also modulated by the pH of the medium <ref name="8a"/>. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8 <ref name="8a"/> <ref name="6a">PMID:17240979</ref>. | ||
| - | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin T, in ''Mycobacterium tuberculosis'' (Figure 1)<ref name= "5a">PMID:22607697</ref>. Mycobactin T is synthesized by the proteins encoded by the ''mbt'' and ''mbt2'' gene clusters <ref name="5a"/>. The gene Rv2386c is essential for the in vitro growth of ''M. tuberculosis'' and codes the enzyme MbtI <ref name="1a">PMID:20512795</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. <ref name="8a"/> | + | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin T, in ''Mycobacterium tuberculosis'' (Figure 1)<ref name= "5a">PMID:22607697</ref>. Mycobactin T is synthesized by the proteins encoded by the ''mbt'' and ''mbt2'' gene clusters <ref name="5a"/>. The gene Rv2386c is essential for the in vitro growth of ''M. tuberculosis'' and codes the enzyme MbtI <ref name="1a">PMID:20512795</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. <ref name="8a"/> <ref name="7a">PMID:10655517</ref><ref name="3a">PMID:16923875</ref> Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action <ref name= "1a"> PMID:20512795</ref> <ref name= "2a">PMID:23108268</ref> <ref name= "7a">PMID:10655517</ref> <ref name= "5a"/> <ref name= "4a">DOI:10.1021/bi2009739</ref> <ref name= "9a">PMID:14982443</ref> |
[[Image:Pathways.png|500 px|center|thumb|'''Figure 1:''' Pathways catalyzed by wild-type MbtI<ref name= "8a">PMID:22307014</ref>.]] | [[Image:Pathways.png|500 px|center|thumb|'''Figure 1:''' Pathways catalyzed by wild-type MbtI<ref name= "8a">PMID:22307014</ref>.]] | ||
| Line 24: | Line 24: | ||
MbtI structure has a mobile element (residues 268-293 and 324-336) that can adopt a <scene name='69/694235/Irp9_closed_state/2'>closed</scene> or <scene name='69/694235/2g5f_with_open_loop/1'>open conformation</scene> depending on whether or not ligands are bound to the active site.<ref name="3a"/> The closed conformation partially obstructs the active site. <ref name= "5a"/>. | MbtI structure has a mobile element (residues 268-293 and 324-336) that can adopt a <scene name='69/694235/Irp9_closed_state/2'>closed</scene> or <scene name='69/694235/2g5f_with_open_loop/1'>open conformation</scene> depending on whether or not ligands are bound to the active site.<ref name="3a"/> The closed conformation partially obstructs the active site. <ref name= "5a"/>. | ||
| - | Inhibition studies have also shown a switch in binding mode at the MbtI active site for inhibitors carrying a substituted enolpyruvyl group compared to the chorismate substrate | + | Inhibition studies have also shown a switch in binding mode at the MbtI active site for inhibitors carrying a substituted enolpyruvyl group compared to the chorismate substrate <ref name="5a">PMID:22607697</ref> <ref name= |
| + | "2a">PMID:23108268</ref> <ref name="1a">PMID:20512795</ref>. Crystal structures and fluorescent-based thermal shift assays show that substituents larger than a methyl group are accommodated in the active site of MbtI through localized flexibility in the peptide backbone.<ref name="5a"/> Positioning of the <scene name='69/694235/3st6_structure_bindingpocket/3'>active site residues</scene> of MbtI in [[3ST6]] with the inhibitor AMT bound is highly similar to the positioning of the <scene name='69/694235/3log_bindingpocket/3'>active site residues</scene> in closed form of MbtI [[3log]] with succinic acid bound <ref name= "5a"/>. The AMT inhibitor contains an unmodified enolpyruvyl side chain and resembles the structure of the natural substrate, chorismate. [[3log]] and [[3ST6]] are shown to share a similar binding mode, termed binding mode 1. Isochorismate inhibitors with modified enolpyruvl side chains ([[3VEH]], [[3RV9]], [[3RV8]], [[3RV7]], [[3RV6]]) utilize a novel binding mode, termed mode 2, which involves the <scene name='69/694235/3veh_structure_bindingpocket2/2'>reorientation of the isochorismate analogue within the active site</scene>. Movement of the peptide backbone away from the closed form of MbtI is required to accommodate the enolpyruyl modified inhibitors. | ||
==Molecular Mechanism== | ==Molecular Mechanism== | ||
| Line 66: | Line 67: | ||
==Inhibition Studies== | ==Inhibition Studies== | ||
| - | MbtI Inhibition studies aid in the future design of [http://psychology.wikia.com/wiki/Antitubercular_drugs anti-tubercular agents] and [http://en.wikipedia.org/wiki/Broad-spectrum_antibiotic broad-spectrum antibiotics] with a novel mode of action. Mimics of the enzyme-bound intermediate of MbtI, <scene name='69/694235/3sr6_inhibitor/3'>isochorismate</scene>, prove to be significantly more potent inhibitors than mimics of the substrate, chorismate <ref name= "1a"/>. The isochorismate mimic based on a 2,3-dihydroxybenzoate scaffold showed low-micromolar inhibition constants against MbtI that were an order of magnitude more potents than the natural substrates. The most potent inhibitors contained hydrophobic enol ether side chains at C3 instead of the enol-pyruvyl side chains seen in chorismate and isochorismate | + | MbtI Inhibition studies aid in the future design of [http://psychology.wikia.com/wiki/Antitubercular_drugs anti-tubercular agents] and [http://en.wikipedia.org/wiki/Broad-spectrum_antibiotic broad-spectrum antibiotics] with a novel mode of action. Mimics of the enzyme-bound intermediate of MbtI, <scene name='69/694235/3sr6_inhibitor/3'>isochorismate</scene>, prove to be significantly more potent inhibitors than mimics of the substrate, chorismate <ref name= "1a"/>. The isochorismate mimic based on a 2,3-dihydroxybenzoate scaffold showed low-micromolar inhibition constants against MbtI that were an order of magnitude more potents than the natural substrates. The most potent inhibitors contained hydrophobic enol ether side chains at C3 instead of the enol-pyruvyl side chains seen in chorismate and isochorismate. <ref name="1a">PMID:20512795</ref> Increased potency of inhibitors with a substituted enolpyruvyl group has been attributed to a change in the binding mode through localized flexibility of the peptide backbone. |
Two binding mode at the MbtI active site have been observed based on the structure of the inhibitor. | Two binding mode at the MbtI active site have been observed based on the structure of the inhibitor. | ||
Revision as of 22:26, 26 April 2015
Contents |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Manos-Turvey A, Bulloch EM, Rutledge PJ, Baker EN, Lott JS, Payne RJ. Inhibition studies of Mycobacterium tuberculosis salicylate synthase (MbtI). ChemMedChem. 2010 Jul 5;5(7):1067-79. PMID:20512795 doi:10.1002/cmdc.201000137
- ↑ 3.0 3.1 Lamb AL. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug, 12. PMID:21823653 doi:http://dx.doi.org/10.1021/bi2009739
- ↑ 4.0 4.1 4.2 4.3 De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1252-7. PMID:10655517
- ↑ Zwahlen J, Kolappan S, Zhou R, Kisker C, Tonge PJ. Structure and mechanism of MbtI, the salicylate synthase from Mycobacterium tuberculosis. Biochemistry. 2007 Jan 30;46(4):954-64. PMID:17240979 doi:10.1021/bi060852x
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Harrison AJ, Yu M, Gardenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS. The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol. 2006 Sep;188(17):6081-91. PMID:16923875 doi:http://dx.doi.org/188/17/6081
- ↑ 8.0 8.1 8.2 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ 9.0 9.1 He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
Student contributors
Stephanie Raynor and Robin Gagnon
Related pdb files and proteopedia pages
3D structures of isochorismate pyruvate lyase
3log – MtIPL/isochorismate synthase - Mycobacterium tuberculosis
3rv6, 3rv7, 3rv8, 3rv9, 3st6, 3veh - MtIPL/isochorismate synthase + inhibitor
2h9c – PaIPL residues 1-99 – Pseudomonas aeruginosa
2h9d - PaIPL + pyruvate
3LOG
3D structure of isochorismate synthase
2eua, 3bzm, 3bzn - MenF from E. coli
3os6 - DhbC from Bacillus anthracis
3gse - MenF from Yersinia pestis
3hwo - EntC
3D structure of salicylate synthase
3veh - MbtI with inhibitor methylAMT
3st6 - MbtI with isochorismate analogue inhibitor
3rv6 (Phenyl R-group), 3rv7 (Isopropyl R-group), 3rv8 (Cyclopropyl R-group), 3rv9 (Ethyl R-group) - MbtI with inhibitor
2fn0, 2fn1 (with products salicylate and pyruvate) - Irp9 from Yersinia enterocolitica
2i6y - MbtI