Sandbox Reserved 1066
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | '''Salicylate synthase (SS)''' | |
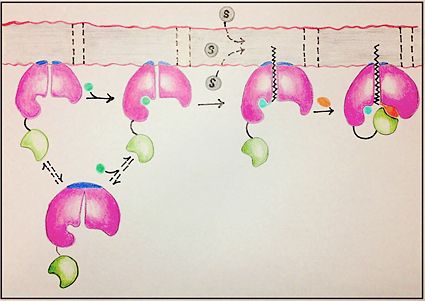
| - | Chromate is converted to salicylate synthase by MbtI through an intermediate isochromate | + | Chromate is converted to salicylate synthase and pyruvate by MbtI through an intermediate isochromate. The pyruvate molecule is expelled after the intermediate step and salicylate is incorporated in the biosynthesis of mycobactin T (Figure 9,10)<ref name="2a">PMID:23108268</ref>. Inhibition studies revealed two binding modes of MbtI based on the structure of the substrate <ref name="5a"/>. Mimics of isochromate inhibitors with modified enolpyruvly side chains showed the greatest inhibition capability and reoriented the substrate within the active side of the enzyme causing the backbone of the enzyme to shift away from the closed conformation (Figure 3,4,5)<ref name="5a"/>. A clear mechanism for the salicylate synthase activity of MbtI is currently unknown<ref name="5a"/>. |
| + | |||
| + | [[Image:Image:Salicylate synthase chem draw.png]] | ||
<blockquote></blockquote>{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <blockquote></blockquote>{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
Revision as of 00:11, 27 April 2015
Salicylate synthase (SS) Chromate is converted to salicylate synthase and pyruvate by MbtI through an intermediate isochromate. The pyruvate molecule is expelled after the intermediate step and salicylate is incorporated in the biosynthesis of mycobactin T (Figure 9,10)[1]. Inhibition studies revealed two binding modes of MbtI based on the structure of the substrate [2]. Mimics of isochromate inhibitors with modified enolpyruvly side chains showed the greatest inhibition capability and reoriented the substrate within the active side of the enzyme causing the backbone of the enzyme to shift away from the closed conformation (Figure 3,4,5)[2]. A clear mechanism for the salicylate synthase activity of MbtI is currently unknown[2].
Image:Image:Salicylate synthase chem draw.png
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
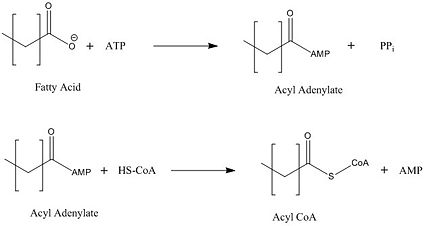
Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase
| |||||||||||
References
- ↑ Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named5a - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012
- ↑ Jatana N, Jangid S, Khare G, Tyagi AK, Latha N. Molecular modeling studies of Fatty acyl-CoA synthetase (FadD13) from Mycobacterium tuberculosis--a potential target for the development of antitubercular drugs. J Mol Model. 2011 Feb;17(2):301-13. doi: 10.1007/s00894-010-0727-3. Epub 2010 May, 8. PMID:20454815 doi:http://dx.doi.org/10.1007/s00894-010-0727-3
- ↑ 5.0 5.1 5.2 5.3 5.4 Khare G, Gupta V, Gupta RK, Gupta R, Bhat R, Tyagi AK. Dissecting the role of critical residues and substrate preference of a Fatty Acyl-CoA Synthetase (FadD13) of Mycobacterium tuberculosis. PLoS One. 2009 Dec 21;4(12):e8387. doi: 10.1371/journal.pone.0008387. PMID:20027301 doi:10.1371/journal.pone.0008387
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Jatana N, Jangid S, Khare G, Tyagi AK, Latha N. Molecular modeling studies of Fatty acyl-CoA synthetase (FadD13) from Mycobacterium tuberculosis--a potential target for the development of antitubercular drugs. J Mol Model. 2011 Feb;17(2):301-13. doi: 10.1007/s00894-010-0727-3. Epub 2010 May, 8. PMID:20454815 doi:http://dx.doi.org/10.1007/s00894-010-0727-3
- ↑ 7.0 7.1 7.2 Schroeder EK, de Souza N, Santos DS, Blanchard JS, Basso LA. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis. Curr Pharm Biotechnol. 2002 Sep;3(3):197-225. PMID:12164478
Similar Proteopedia Pages
Phosphopantetheinyl Transferase
External Resources
Lipids Wikipedia page
Tuberculosis Wikipedia page
Mycobacterium tuberculosis Wikipedia page
Coenzyme A Wikipedia page
Acyl CoA Wikipedia Page
Mycolic Acid Wikipedia page
Peripheral Membrane Protein Wikipedia page
Ethionamide Wikipedia page
Isoniazid Wikipedia page
Thiocarlide Wikipedia page
Pyrazinamide Wikipedia page