We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Michael Roberts/BIOL115 Myo
From Proteopedia
< User:Michael Roberts(Difference between revisions)
m |
|||
| Line 41: | Line 41: | ||
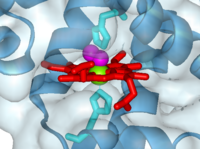
'''PROXIMAL AND DISTAL HISTIDINES''': The iron atom sits either side of the side chains of two <scene name='User:Michael_Roberts/BIOL115_Myo/Heme/4'>histidine residues</scene>. | '''PROXIMAL AND DISTAL HISTIDINES''': The iron atom sits either side of the side chains of two <scene name='User:Michael_Roberts/BIOL115_Myo/Heme/4'>histidine residues</scene>. | ||
| - | One of these (coloured cyan) is attached to the iron atom, and is known as the ''proximal'' histidine. It is also referred to as His F8, because it is the eighth residue of helix F. The other (green) is called the ''distal'' histidine, also referred to as | + | One of these (coloured cyan) is attached to the iron atom, and is known as the ''proximal'' histidine. It is also referred to as His F8, because it is the eighth residue of helix F. The other (green) is called the ''distal'' histidine, also referred to as His E7 (7<sup>th</sup> residue of helix E). |
| - | Note how the iron is pulled out slightly to one side of the plane of the | + | Note how the iron is pulled out slightly to one side of the plane of the haem group as a result of it's co-ordination with the side chain of the proximal histidine. |
'''OXYGEN''': | '''OXYGEN''': | ||
The space between the iron and the distal histidine is where the <scene name='User:Michael_Roberts/BIOL115_Myo/Heme/6'>oxygen</scene> (pink) binds. | The space between the iron and the distal histidine is where the <scene name='User:Michael_Roberts/BIOL115_Myo/Heme/6'>oxygen</scene> (pink) binds. | ||
| - | Note the angled orientation of the oxygen relative to the plane of the | + | Note the angled orientation of the oxygen relative to the plane of the haem. The oxygen-haem complex is stabilised by the presence of the side chain of the distal His, which contributes a hydrogen atom that hydrogen bonds with the O<sub>2</sub>. The presence of the distal His also reduces the affinity of haem for carbon monoxide, by displacing it from it's more natural position perpendicular to the plane of the haem into a more angled position, similar to bound O<sub>2</sub>. |
</StructureSection> | </StructureSection> | ||
Current revision
Myoglobin with oxygen bound to heme (1a6m)
The heme group and oxygen binding in myoglobin.
Myoglobin is a protein whose function is to store oxygen in muscle tissues. Like heamoglobin, it is red in colour, and it is myoglobin that gives muscle its strong red colour.
Myoglobin was the first globular protein for which the 3-dimensional structure was solved, back in the late 1950s. It gives its name to the 'globin fold', a common alpha domain motif. An alpha domain is a structural region composed entirley of alpha-helix.
Click on the 'green links' in the text in the scrollable section below to examine this molecule in more detail.
| |||||||||||
