Sandbox Reserved 1078
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}} | + | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
| - | + | == Background == | |
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: ag85A, B, and C. The ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. <ref>PMID: 10655617</ref> Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant mycotaberia tuberculosis. <ref>PMID: 10200974</ref> | ||
==Structure== | ==Structure== | ||
| - | + | <StructureSection load='1dqz' size='400' side='right' caption='Antigen 85C in Mycobacterium Tuberculosis' scene=''> | |
| - | + | ||
| + | == General Structure == | ||
| - | = | + | Antigen 85C was crystallized in its dimeric form.<ref>PMID: 25028518</ref> The <scene name='69/694220/Secondary_structures/2'>secondary structure</scene> shown in the monomeric form is composed of helices with one interwoven beta sheet. Due to the serine hydrolase activity of Ag85C, the enzyme contains a ‡/? hydrolase fold with a central ?-sheet bordered by ‡?helices, and this tertiary conformation is highly conserved across enzymes that function in this capacity. <ref>PMID:10655617</ref> The substrate binding pocket of Ag85C is composed of two separate but equally important components; there is carbohydrate binding pocket for the trehalose, and there is a fatty acid binding pocket for the mycolic acid. As a result, trahalose monomycolate can effectively bind to the Ag85C binding pocket. |
| - | '' | + | |
| - | + | == Enzymatic Activity == | |
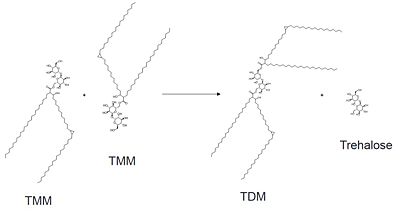
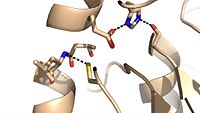
| + | Mutagenesis studies have confirmed the Ag85C functions through a Glu-His-Ser <scene name='69/694220/Catalytic_triad/2'>catalytic triad</scene>, similar to that of chymotrypsin. By modifying each of the catalytic residues separately testing the enzyme?s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity (Figure #). The S124 alcohol?s nucleophilicity is inductively strengthened through H260 and E224, which allows S124 to hydrolyze trehalose 6, 6?-dimycolate. The formation of the functional catalytic triad relies on upon Van der Waals interaction between C209 and the peptide bond between L232 and T231. This interaction results in a kinked conformation of the ‡9 helix, which promotes that activity of the catalytic triad. As a result, Ag85C, a mycolyl transferase, can facilitate the modification of trehalose monomycolates to trehalose dimycolates, which are then transported to the bacterial cell wall. | ||
| - | [[Image: | + | [[Image:General_mechanism.jpg|400 px|center|General reaction catalyzed by Antigen 85C]] |
| + | == Methods of Inhibition == | ||
| - | + | [[Image:C209.jpeg|200 px|left|thumb|Cys209 stabilizing kinked formation of alpha-9 helix]] | |
| - | + | ||
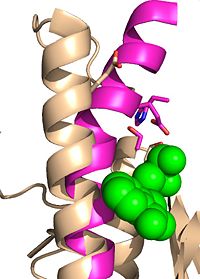
| - | + | Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the mycobacteria tuberculosis cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between C209 and L232-T 231 (Figure #). The C209 facilitated interaction causes the <scene name='69/694220/Alpha_9_helix/2'>‡9 helix</scene> to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition.> | |
| - | ==Molecular Mechanism== | ||
| - | '''Magnesium cation effect''' | ||
| - | + | [[Image:Ebselen_inhibition.jpeg|200 px|right|thumb|Ebselen inhibition relaxing the alpha-9 helix]] | |
| - | '''Isochorismate pyruvae lyase (IPL)''' | ||
| - | + | Ag85C can be inhibited by ebselen covalently bound to the sulfur of C209. Ebselen is a thiol-modifying agent that serves as an electrophile for the C209 that results in a sulfur-selenium bond. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T31, which effectively disrupts the interaction that holds the ‡9 helix in the active conformation. Furthermore, the bulk of ebselen creates steric hindrance with the ‡9 helix residues (Figure #). Relaxation of the ‡9 helix removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues decreases the nucleophilicity of the S124 alcohol which decreases serine hydrolytic activity. | |
| - | + | ===Inhibitors=== | |
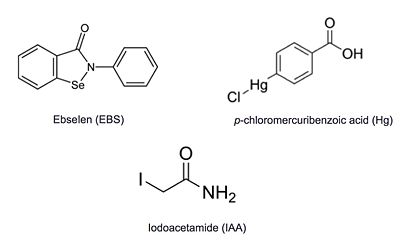
| - | + | Additional thiol-modifying agents, p-chloromercuribenzoic acid and iodoacetamide, were crystalized with Ag85C. The structures show that each of these thiol-reactive inhibitors covalently bound to C209 and caused a relaxation of the ‡9 helix in a similar fashion to ebselen. | |
| + | |||
| + | [[Image:Inhibitors_Ag85c.jpeg|400 px|center]] | ||
| - | + | </StructureSection> | |
| - | A magnesium ion in the active site orients the C1 carboxyl group of chorismate. A lysine residue then serves as a general base for the activation of a water molecule to attack at C2. The catalytic mechanism for conversion of isochorismate to salicylate by MbtI is a sigmatropic, pericyclic mechanism that is pH-dependent. Chromate mutase activity is only observed in the absence of magnesium ion in the active site while salicylate synthase activity is depended on magnesium ion. The active site of MbtI is altered by the removal of the magnesium cofactor causing chromate mutase activity. MbtI has differing binding modes for chromate that leads to different substrate conformations/transition states and resulting in different products. | ||
| - | |||
| - | ==Inhibition Studies== | ||
| - | |||
| - | MbtI Inhibition studies aid in the future design of anti-tubercular agents and broad-spectrum antibiotics. Mimics of the enzyme-bound intermediate of MbtI, isochorismate, prove to be significantly more potent inhibitors than the substrate, chorismate mimics <ref name= "manos-turvey"/>. Specifically, <scene name='69/694235/3rv6_with_vae1/1'>2-hydroxybenzoate-based inhibitors</scene> that contain extended hydrophobic enol ether side chains at C3 in place of the enol-pyruvate side chain found in chorismate and isochorismate. | ||
| - | |||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
| - | + | <references/> | |
Revision as of 14:13, 30 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Background
The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: ag85A, B, and C. The ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. [1] Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant mycotaberia tuberculosis. [2]
Structure
| |||||||||||
References
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413
- ↑ Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999 Mar;31(5):1573-87. PMID:10200974
- ↑ Favrot L, Lajiness DH, Ronning DR. Inactivation of the Mycobacterium tuberculosis Antigen 85 complex by covalent, allosteric inhibitors. J Biol Chem. 2014 Jul 14. pii: jbc.M114.582445. PMID:25028518 doi:http://dx.doi.org/10.1074/jbc.M114.582445
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413