We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Malate dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 53: | Line 53: | ||
**[[1civ]] – MDH+NADP – ''Flaveria bidentis''<br /> | **[[1civ]] – MDH+NADP – ''Flaveria bidentis''<br /> | ||
**[[1b8u]], [[1b8v]] – AaMDH+NAD - ''Aquaspirillum arcticum''<br /> | **[[1b8u]], [[1b8v]] – AaMDH+NAD - ''Aquaspirillum arcticum''<br /> | ||
| - | **[[5mdh]] – | + | **[[5mdh]] – pMDH+NAD+alpha-ketomalonic acid – pig<br /> |
| - | **[[4mdh]] – | + | **[[4mdh]] – pMDH+NAD<br /> |
| - | **[[4i1i]] – LmMd + NAD – ''Leishmania major'' | + | **[[4i1i]] – LmMd + NAD – ''Leishmania major''<br /> |
| + | **[[4plh]], [[4plt]] – ApMd + NAD + oxamate – ''Apicomplexa''<br /> | ||
| + | **[[4plv]], [[4plw]] – ApMd + NAD + lactate <br /> | ||
| + | **[[4ply]] – ApMd + NAD + malate <br /> | ||
| + | **[[4ros]] – MeMd + oxaloacetate + ADPR – ''Methylobacterium extorquens'' <br /> | ||
* apo-MDH | * apo-MDH | ||
| Line 70: | Line 74: | ||
**[[1b8p]] – AaMDH <br /> | **[[1b8p]] – AaMDH <br /> | ||
**[[7mdh]] – MDH – Sorgum bicolor<br /> | **[[7mdh]] – MDH – Sorgum bicolor<br /> | ||
| - | **[[1mld]] – | + | **[[1mld]] – pMDH<br /> |
**[[2cmd]] - EcMd+citrate<br /> | **[[2cmd]] - EcMd+citrate<br /> | ||
**[[3nep]] – Md – ''Salinibacter ruber''<br /> | **[[3nep]] – Md – ''Salinibacter ruber''<br /> | ||
| Line 79: | Line 83: | ||
**[[4bgt]] – CaMd <br /> | **[[4bgt]] – CaMd <br /> | ||
**[[4cl3]] – ChaMd <br /> | **[[4cl3]] – ChaMd <br /> | ||
| + | **[[4bgu]] – Md – ''Haloferax volcanii''<br /> | ||
| + | **[[4bgv]] – Md – ''Picrophilus torridus''<br /> | ||
| + | **[[4ror]] – MeMd <br /> | ||
| + | **[[4tvo]] – Md – ''Mycobacterium tubrtculosis'' <br /> | ||
| + | |||
}} | }} | ||
==Additional Resources== | ==Additional Resources== | ||
Revision as of 09:25, 4 May 2015
| |||||||||||
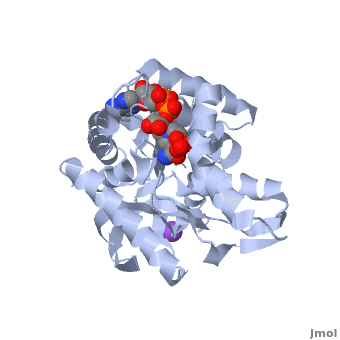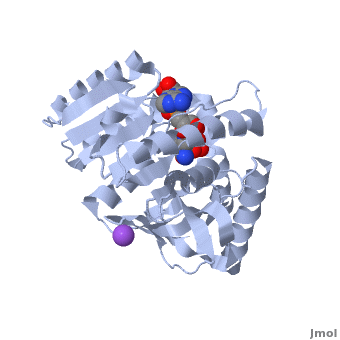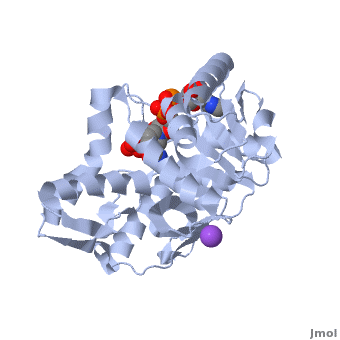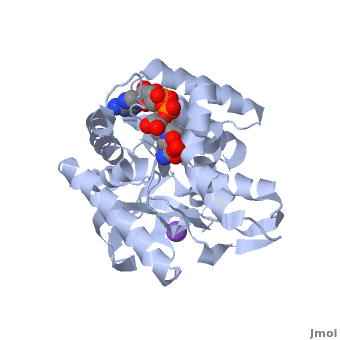
3D Structures of Malate Dehydrogenase
Updated on 04-May-2015
The holo-MDH contains NAD or its derivatives while the apo-MDH lacks it.
Additional Resources
References
- ↑ Minarik P, Tomaskova N, Kollarova M, Antalik M. Malate dehydrogenases--structure and function. Gen Physiol Biophys. 2002 Sep;21(3):257-65. PMID:12537350
- ↑ Matsuda T, Takahashi-Yanaga F, Yoshihara T, Maenaka K, Watanabe Y, Miwa Y, Morimoto S, Kubohara Y, Hirata M, Sasaguri T. Dictyostelium Differentiation-Inducing Factor-1 Binds to Mitochondrial Malate Dehydrogenase and Inhibits Its Activity. J Pharmacol Sci. 2010 Feb 20. PMID:20173310
- ↑ Matsuda T, Takahashi-Yanaga F, Yoshihara T, Maenaka K, Watanabe Y, Miwa Y, Morimoto S, Kubohara Y, Hirata M, Sasaguri T. Dictyostelium Differentiation-Inducing Factor-1 Binds to Mitochondrial Malate Dehydrogenase and Inhibits Its Activity. J Pharmacol Sci. 2010 Feb 20. PMID:20173310
- ↑ Musrati RA, Kollarova M, Mernik N, Mikulasova D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998 Sep;17(3):193-210. PMID:9834842
- ↑ Boernke WE, Millard CS, Stevens PW, Kakar SN, Stevens FJ, Donnelly MI. Stringency of substrate specificity of Escherichia coli malate dehydrogenase. Arch Biochem Biophys. 1995 Sep 10;322(1):43-52. PMID:7574693 doi:http://dx.doi.org/10.1006/abbi.1995.1434
- ↑ Plancarte A, Nava G, Mendoza-Hernandez G. Purification, properties, and kinetic studies of cytoplasmic malate dehydrogenase from Taenia solium cysticerci. Parasitol Res. 2009 Jul;105(1):175-83. Epub 2009 Mar 10. PMID:19277715 doi:10.1007/s00436-009-1380-6
- ↑ Goward CR, Nicholls DJ. Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci. 1994 Oct;3(10):1883-8. PMID:7849603 doi:http://dx.doi.org/10.1002/pro.5560031027
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Jake Ezell, Joel L. Sussman, Joshua Johnson, Angel Herraez, Jaime Prilusky, David Canner