Sandbox 985
From Proteopedia
| Line 1: | Line 1: | ||
| - | MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin. | + | '''Matrix metalloproteinase 9''' |
| + | |||
| + | MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin. | ||
| + | |||
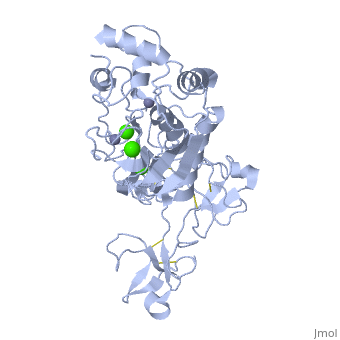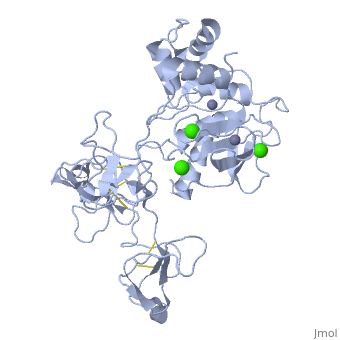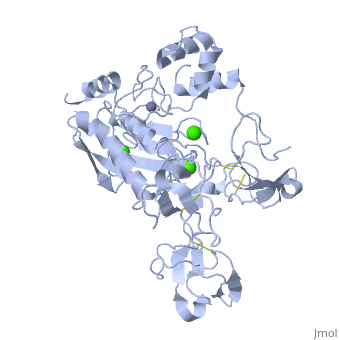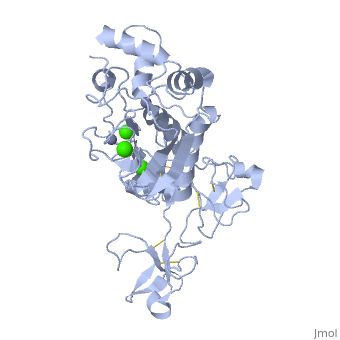
<StructureSection load='1l6j' size='340' side='right' caption='Human matrix metalloproteinase MMP9 (gelatinase B),1l6j' scene=''> | <StructureSection load='1l6j' size='340' side='right' caption='Human matrix metalloproteinase MMP9 (gelatinase B),1l6j' scene=''> | ||
| - | This is a default text for your page '''Matrix metalloproteinase 9'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
== Function == | == Function == | ||
MMP9 is involved in inflammatory responses, tissue remodeling, wound healing, tumor growth and metastasis. MMP9 may also play an important part in local proteolysis of the extracellular matrix and in leukocyte migration, as well as in bone osteoclastic resorption. | MMP9 is involved in inflammatory responses, tissue remodeling, wound healing, tumor growth and metastasis. MMP9 may also play an important part in local proteolysis of the extracellular matrix and in leukocyte migration, as well as in bone osteoclastic resorption. | ||
| + | |||
| + | Emerging evidences suggest that membrane-anchored proteases play important roles during growth, morphogenesis, tissue repair, and pathogenesis. It has been proposed that, by concentrating at or near the cell surface, the proteolytic activities of these enzymes can be effective even in the presence of high concentrations of (diffusable) inhibitors.<ref></Takahashi, C. "Regulation of Matrix Metalloproteinase-9 and Inhibition of Tumor Invasion by the Membrane-anchored Glycoprotein RECK." Proceedings of the National Academy of Sciences 95.22 (1998): 13221-3226. Web. 4 May 2015.> | ||
| + | |||
MMP-9 is absent from most normal adult tissues, including the intestinal epithelial cells. We and several others have shown that MMP-9 is highly expressed during intestinal inflammation in different animal models and human IBD. In addition to its role in inflammation, recent studies from our laboratory have shown that MMP-9 plays a role in epithelial cell differentiation. | MMP-9 is absent from most normal adult tissues, including the intestinal epithelial cells. We and several others have shown that MMP-9 is highly expressed during intestinal inflammation in different animal models and human IBD. In addition to its role in inflammation, recent studies from our laboratory have shown that MMP-9 plays a role in epithelial cell differentiation. | ||
| + | |||
| + | == Structure == | ||
| + | MMP9 is composed of the following domains: a gelatin-binding domain consisting of three fibronectin type II units, a catalytic domain containing the zinc-binding site, a proline-rich type V collagen-homologous domain and a hemopexin-like domain. The zinc binding motif HEXXHXXGXXH in the catalytic domain, and the “cysteine switch” motif PRCGXPD in the propeptide are common structural signatures, where three histidines in the zinc binding motif coordinate and the cysteine in the propetide coordinate with the catalytic zinc ion. MMP9 is produced by the several cell types, normal alveolar macrophages and granulocytes. | ||
== Disease == | == Disease == | ||
| Line 24: | Line 31: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | < | + | <references/> |
Revision as of 17:08, 4 May 2015
Matrix metalloproteinase 9
MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin.
| |||||||||||
References
- ↑ </Takahashi, C. "Regulation of Matrix Metalloproteinase-9 and Inhibition of Tumor Invasion by the Membrane-anchored Glycoprotein RECK." Proceedings of the National Academy of Sciences 95.22 (1998): 13221-3226. Web. 4 May 2015.>
MMP-9 is absent from most normal adult tissues, including the intestinal epithelial cells. We and several others have shown that MMP-9 is highly expressed during intestinal inflammation in different animal models and human IBD. In addition to its role in inflammation, recent studies from our laboratory have shown that MMP-9 plays a role in epithelial cell differentiation.
Contents
Structure
MMP9 is composed of the following domains: a gelatin-binding domain consisting of three fibronectin type II units, a catalytic domain containing the zinc-binding site, a proline-rich type V collagen-homologous domain and a hemopexin-like domain. The zinc binding motif HEXXHXXGXXH in the catalytic domain, and the “cysteine switch” motif PRCGXPD in the propeptide are common structural signatures, where three histidines in the zinc binding motif coordinate and the cysteine in the propetide coordinate with the catalytic zinc ion. MMP9 is produced by the several cell types, normal alveolar macrophages and granulocytes.
Disease
Cardiovascular
Matrix metalloproteinase (MMP)-9, one of the most widely investigated MMPs, regulates pathological remodeling processes that involve inflammation and fibrosis in cardiovascular disease. MMP-9 directly degrades extracellular matrix (ECM) proteins and activates cytokines and chemokines to regulate tissue remodeling. MMP-9 deletion or inhibition has proven overall beneficial in multiple animal models of cardiovascular disease.
Hypertension
MMP-9 activity is induced very early with the development of hypertension, contributing to collagen breakdown and arterial distensibility. An increase in fibrillar collagen in the compensated stage of hypertension is associated with increased MMP-9 activity. An increased arterial pressure and altered remodeling in the blood vessels lead to a pressure overload of the heart. Under these conditions, both vascular and cardiac tissues undergo additional compensatory remodeling. MMP-9 activity is increased in arteries with high pressure compared with vessels under normal pressure.
Regulation
Activators: Ca2+ and Zn2+
Gelatinase B activity is under strict control at various levels: transcription of the gene by cytokines and cellular interactions; activation of the pro-enzyme by a cascade of enzymes comprising serine proteases and other MMPs; and regulation by specific tissue inhibitors of MMPs (TIMPs) or by unspecific inhibitors, such as alpha2-macroglobulin.<ref> </Opdenakker, G., PE Van Der Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. "Gelatinase B Functions as Regulator and Effector in Leukocyte Biology." Gelatinase B Functions as Regulator and Effector in Leukocyte Biology 69.6 (2001): 851-59. Gelatinase B Functions as Regulator and Effector in Leukocyte Biology. Journal of Leukocyte Biology, June 2001. Web. 04 May 2015.>
</li></ol></ref>