We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
PLC beta 3 Gq
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structural highlights == | == Structural highlights == | ||
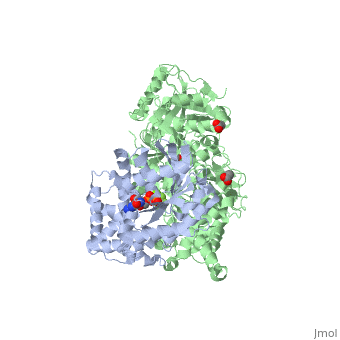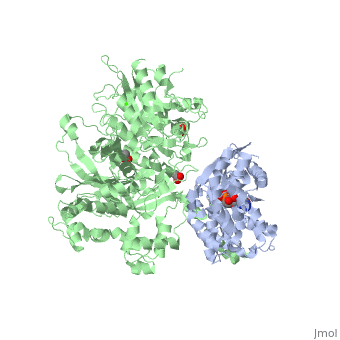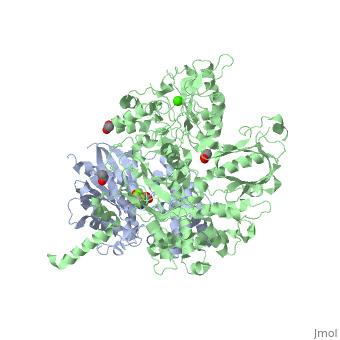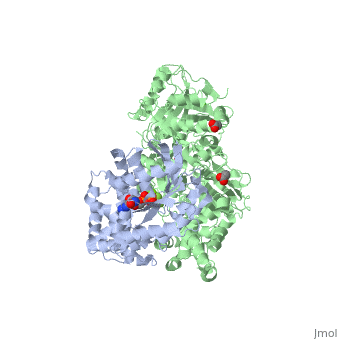
The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called <scene name='70/701452/Fig2/2'>switch regions I-III</scene>. | The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called <scene name='70/701452/Fig2/2'>switch regions I-III</scene>. | ||
| - | |||
<scene name='70/701452/Fig1/9'>The PLC- β3 has several domains</scene> consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel that the X/Y linker connect its two halves and a C2 domain. | <scene name='70/701452/Fig1/9'>The PLC- β3 has several domains</scene> consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel that the X/Y linker connect its two halves and a C2 domain. | ||
PLC- β3 engages Gαq throughout three regions. First, an extended loop between the third and fourth EF hands of PLC- β3 directly buttresses switch residues critical for GTP hydrolysis by Gαq. Second, the region of PLC- β3 that connects the catalytic TIM barrel and the C2 domain interacts with both switches 1 and 2 of Gαq. Third, a segment composed of a helix-turn-helix at the C terminus of the C2 domain resides primarily within a shallow declivity on the surface of Gαq formed by switch 2 and α3. | PLC- β3 engages Gαq throughout three regions. First, an extended loop between the third and fourth EF hands of PLC- β3 directly buttresses switch residues critical for GTP hydrolysis by Gαq. Second, the region of PLC- β3 that connects the catalytic TIM barrel and the C2 domain interacts with both switches 1 and 2 of Gαq. Third, a segment composed of a helix-turn-helix at the C terminus of the C2 domain resides primarily within a shallow declivity on the surface of Gαq formed by switch 2 and α3. | ||
Revision as of 17:49, 6 May 2015
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
| |||||||||||
References
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403