We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1051
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == Background == | ||
| - | + | =Antigen 85C from ''Mycobacterium tuberculosis''= | |
| - | == | + | ==Introduction== |
| + | |||
<StructureSection load='1dqz' size='400' side='right' caption='Antigen 85C in ''Mycobacterium Tuberculosis''' scene=''> | <StructureSection load='1dqz' size='400' side='right' caption='Antigen 85C in ''Mycobacterium Tuberculosis''' scene=''> | ||
| - | == | + | == Background == |
| + | The antigen 85 (ag85) complex in [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''], which is responsible for causing the disease [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], is composed of three intracellular membrane proteins: Ag85A, B, and C. The Ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. <ref>PMID: 10655617</ref> The cell wall of ''Mycobacterium tuberculosis'' is composed of three primary molecules: [http://en.wikipedia.org/wiki/Peptidoglycan peptidoglycans], [http://en.wikipedia.org/wiki/Arabinogalactan arbinogalactans], and [http://en.wikipedia.org/wiki/Mycolic_acid mycolic acids]. Ag85C is of particular interest due to its transfer of mycolic acids, which is one of the major components in determining cell wall integrity. The mycolic acids are responsible for forming the outermost layer of the cell wall. Mycolic acids have a long fatty acid chain and exhibit extreme hydrophobicity, which effectively creates a hydrophobic envelope surrounding the bacterium. The hydrophobic envelope created by they mycolic acids creates a barrier against small hydrophilic molecules, such as Tuberculosis antibiotics. By targeting this mycoloyltransferase activity, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant ''Mycotaberia tuberculosis''. <ref>PMID: 10200974</ref> | ||
| + | == General Structure == | ||
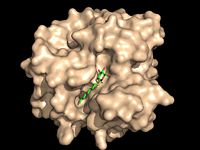
[[Image:Substrate Binding 2D Surface.jpg|200 px|left|thumb|'''Figure 1:''' Octylthioglucoside, a substrate analog, shown in the binding pocket of [http://www.rcsb.org/pdb/explore/explore.do?structureId=1VA5 Ag85C]]] | [[Image:Substrate Binding 2D Surface.jpg|200 px|left|thumb|'''Figure 1:''' Octylthioglucoside, a substrate analog, shown in the binding pocket of [http://www.rcsb.org/pdb/explore/explore.do?structureId=1VA5 Ag85C]]] | ||
Revision as of 18:12, 12 May 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Antigen 85C from Mycobacterium tuberculosis
Introduction
| |||||||||||
References
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413
- ↑ Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999 Mar;31(5):1573-87. PMID:10200974
- ↑ Favrot L, Lajiness DH, Ronning DR. Inactivation of the Mycobacterium tuberculosis Antigen 85 complex by covalent, allosteric inhibitors. J Biol Chem. 2014 Jul 14. pii: jbc.M114.582445. PMID:25028518 doi:http://dx.doi.org/10.1074/jbc.M114.582445
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413