Sandbox WWC4
From Proteopedia
| Line 1: | Line 1: | ||
==Aquaporin (AQP) The Water Channel of the cell== | ==Aquaporin (AQP) The Water Channel of the cell== | ||
| - | < | + | <Structure load='1J4N' size='350' frame='true' align='right' caption='AQP' scene='AQP1' /> |
This is a integral membrane proteins that control the amount of water that travels in and out of the cell<ref name="Takata">PMID:15242101</ref>. Before the discovery of aquaporin, it was thought that water molecule just leaked through the phospholipid bilayer. | This is a integral membrane proteins that control the amount of water that travels in and out of the cell<ref name="Takata">PMID:15242101</ref>. Before the discovery of aquaporin, it was thought that water molecule just leaked through the phospholipid bilayer. | ||
| Line 10: | Line 10: | ||
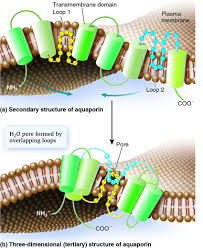
Aquaporin is protien with six transmembrane alpha helices with the amino and craboxyl terminal located in the cytoplasm.<ref name="Murata">PMID:11034202</ref> | Aquaporin is protien with six transmembrane alpha helices with the amino and craboxyl terminal located in the cytoplasm.<ref name="Murata">PMID:11034202</ref> | ||
| + | |||
| + | [[Image:Aquaporin cartoon large.jpg|300px|left|thumb|Water Orientation in AQP]] | ||
-Each monomer is able to channel water. | -Each monomer is able to channel water. | ||
| Line 25: | Line 27: | ||
-If water is flowing outside of the cell then the water molecule is oriented with the oxygen atom facing down. | -If water is flowing outside of the cell then the water molecule is oriented with the oxygen atom facing down. | ||
| - | [[Image: | + | [[Image:Kljgfklgj.jpg|300px|left|thumb|Aquaporin in Membrane]] |
Aquaporin's channel contains asparagines that interact with the hydrogen bonds on the water molecule which allows the orientation of water. | Aquaporin's channel contains asparagines that interact with the hydrogen bonds on the water molecule which allows the orientation of water. | ||
The narrow pore acts to weaken the hydrogen bonds between the water molecules allowing the water to interact with the positively charged arginine, which also acts as a proton filter for the pore.<ref name="Murata" /> | The narrow pore acts to weaken the hydrogen bonds between the water molecules allowing the water to interact with the positively charged arginine, which also acts as a proton filter for the pore.<ref name="Murata" /> | ||
| - | |||
| - | <scene name='69/696849/Aquaporin_with_ligand/1'>colored helix Aquaporin</scene> | ||
---- | ---- | ||
Revision as of 04:40, 14 May 2015
Aquaporin (AQP) The Water Channel of the cell
|
This is a integral membrane proteins that control the amount of water that travels in and out of the cell[1]. Before the discovery of aquaporin, it was thought that water molecule just leaked through the phospholipid bilayer. Aquaporins allow water to flow rapidly to the inside of cell then it would by crossing the bilayer.[1] This protein is highly selective to water molecules , preventing the passage of ions and other solutes.There are multiple types of aquaporins that can allow the transport of other molecules such as glycerol,CO2, ammonia and urea by aquaglyceroporin. It depends on the size of the pore; aquaporin 3 channel has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da[1]. Aquaporins water channel are impermeable to protons and other charged species Water molecules traverse through the pore of the channel in single file[1]. The presence of water channels increases membrane permeability to water[1].
Structure
Aquaporin is protien with six transmembrane alpha helices with the amino and craboxyl terminal located in the cytoplasm.[2]
-Each monomer is able to channel water. -There is a conserved Asn-Pro-Ala sequence that overlap in the middle of the lipid bilayer membrane which creates the'hourglass' structure of the aquaporin. -The hourglass shape allows the water flows, these water pores are completely impermeable to charged species, such as protons. Which is very important to the conservation of membrane's electrochemical potential[1].
Water Enters
Water molecules enter aquaporin and move through the narrow channel by orienting themselves by the electrical field created channel wall.
-The water molecule is oriented with their oxygen atom facing down the channel. -If water is flowing outside of the cell then the water molecule is oriented with the oxygen atom facing down.
Aquaporin's channel contains asparagines that interact with the hydrogen bonds on the water molecule which allows the orientation of water.
The narrow pore acts to weaken the hydrogen bonds between the water molecules allowing the water to interact with the positively charged arginine, which also acts as a proton filter for the pore.[2]
Function
There are thirteen known types of aquaporins in mammals, and six of these are located in the kidney. The most studied aquaporins are AQP1, AQP2, AQP3, and AQP4.The location of Aquaporin is usually found in the kidney, eye in the body. It is found in the basolateral and apical plasma membranes of the proximal tubules and the limb of the loop of Henle in the kidney. Aquaporin are concentrated in the kidney is where there is a need to transporting large amounts of water in and out of the cell. Aquqporin was also found in the plants.
If a person is identified with severe or total deficiency in aquaporin-1. They are generally healthy, they might have a disorder that cause an increase in urine production.
Additionally, it is found in red blood cells, vascular endothelium, the gastrointestinal tract, sweat glands, and lungs.