We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox WWC4
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1h6i' size='350' side='right' caption='Aquaporin' scene='Aquaporin with ligand'> | <StructureSection load='1h6i' size='350' side='right' caption='Aquaporin' scene='Aquaporin with ligand'> | ||
| - | + | Aquqporin (AQP) is a integral membrane proteins that control the amount of water that travels in and out of the cell<ref name="Takata">PMID:15242101</ref>. Before the discovery of aquaporin by Peter Agre (1992); it was thought that water molecules leaked through the phospholipid bilayer one by one. However this could not be the case for cells that released and uptake water such as in the kidneys. | |
Aquaporins allow water to flow rapidly to the inside of cell then it would by crossing the bilayer.<ref name="Takata" /> | Aquaporins allow water to flow rapidly to the inside of cell then it would by crossing the bilayer.<ref name="Takata" /> | ||
This protein is highly selective to water molecules , preventing the passage of ions and other solutes.There are multiple types of aquaporins that can allow the transport of other molecules such as glycerol,CO2, ammonia and urea by aquaglyceroporin. It depends on the size of the pore; aquaporin 3 channel has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da<ref name="Takata" />. Aquaporins water channel are impermeable to protons and other charged species | This protein is highly selective to water molecules , preventing the passage of ions and other solutes.There are multiple types of aquaporins that can allow the transport of other molecules such as glycerol,CO2, ammonia and urea by aquaglyceroporin. It depends on the size of the pore; aquaporin 3 channel has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da<ref name="Takata" />. Aquaporins water channel are impermeable to protons and other charged species | ||
| Line 10: | Line 10: | ||
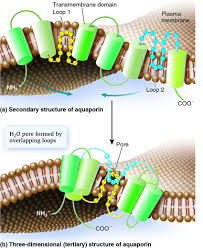
Aquaporin is protien with six transmembrane alpha helices with the amino and craboxyl terminal located in the cytoplasm.<ref name="Murata">PMID:11034202</ref> | Aquaporin is protien with six transmembrane alpha helices with the amino and craboxyl terminal located in the cytoplasm.<ref name="Murata">PMID:11034202</ref> | ||
| - | + | Each monomer is able to channel water.There is a conserved Asn-Pro-Ala sequence that overlap in the middle of the lipid bilayer membrane which creates the'hourglass' structure of the aquaporin.The hourglass shape allows the water flows, these water pores are completely impermeable to charged species, such as protons. Which is very important to the conservation of membrane's electrochemical potential<ref name="Takata" />. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | Which is very important to the conservation of membrane's electrochemical potential<ref name="Takata" />. | + | |
<scene name='69/696849/Chains_of_aquaporin/1'>Chains of Aquaporin (Click to View)</scene> | <scene name='69/696849/Chains_of_aquaporin/1'>Chains of Aquaporin (Click to View)</scene> | ||
Revision as of 05:08, 14 May 2015
Aquaporin (AQP) The Water Channel of the cell
| |||||||||||