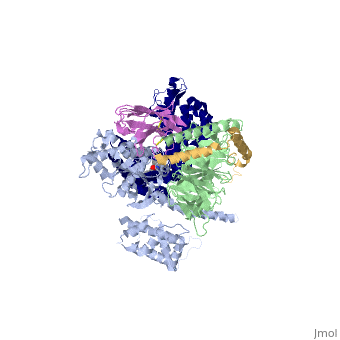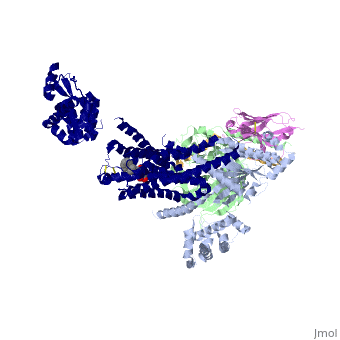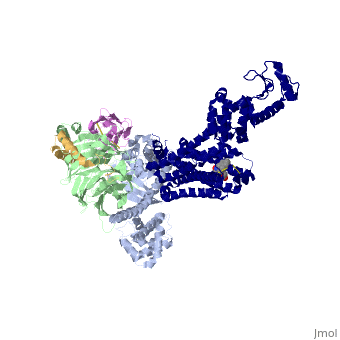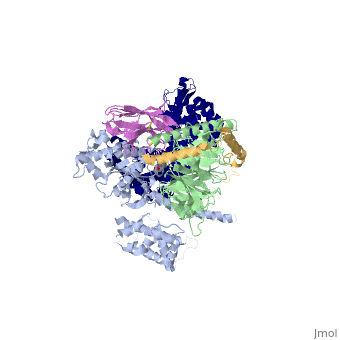
Beta2 adrenergic receptor-Gs protein complex
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
Since these receptors have seven transmembrane helices as well as inner and outer cell regions, they are very difficult to purify and crystalize. Some crystal structures have been determined for the inactive receptors as well as for the G protein that they bind. 3sn6 is the first structure of the full complex of the Beta 2 Adrenergic Receptor bound to Gs in their active state, and it provides the first high-resolution insight into the mechanism of signal transduction across the plasma membrane by a GPCR. | Since these receptors have seven transmembrane helices as well as inner and outer cell regions, they are very difficult to purify and crystalize. Some crystal structures have been determined for the inactive receptors as well as for the G protein that they bind. 3sn6 is the first structure of the full complex of the Beta 2 Adrenergic Receptor bound to Gs in their active state, and it provides the first high-resolution insight into the mechanism of signal transduction across the plasma membrane by a GPCR. | ||
| - | == | + | == G Protein Cycle == |
[[Image:G protein cycle for the b2AR–Gs complex.JPG|left|thumb|G protein cycle for the b2AR–Gs complex. Reprinted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: Nature 477, 549–555, copyright 2011]] | [[Image:G protein cycle for the b2AR–Gs complex.JPG|left|thumb|G protein cycle for the b2AR–Gs complex. Reprinted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: Nature 477, 549–555, copyright 2011]] | ||
| + | The figure shows the G Protein cycle- an extracellular agonist binds to the β2AR leads [[Group:SMART:A Physical Model of the β2-Adrenergic Receptor|to conformational rearrangements of the cytoplasmic ends of transmembrane segments]] that enable the Gs heterotrimer to bind the receptor. GDP is released from the α subunit upon formation of β2AR–Gs complex. The GTP binds to the nucleotide-free α subunit resulting in dissociation of the α and βγ subunits from the receptor. The subunits regulate their respective effector proteins adenylyl cyclase (AC) and Ca2+ channels. The Gs heterotrimer reassembles from α and βγ subunits following hydrolysis of GTP to GDP in the α subunit. b, The purified nucleotide-free β2AR–Gs protein complex maintained in detergent micelles. The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. In the nucleotide-free state, the αAH domain has a variable position relative the αRas domain. | ||
==See Also== | ==See Also== | ||
*[[Adrenergic receptor|Adrenergic receptor]] | *[[Adrenergic receptor|Adrenergic receptor]] | ||
Revision as of 11:07, 17 May 2015
Beta2 adrenergic receptor-Gs protein complex
| |||||||||||
References
Proteopedia Page Contributors and Editors (what is this?)
Dan Elran, Michal Harel, Alexander Berchansky, Joel L. Sussman