User:Rana Saad/The human GABAb receptor
From Proteopedia
| Line 13: | Line 13: | ||
===GBR1 and GBR2 subunits structure=== | ===GBR1 and GBR2 subunits structure=== | ||
Each subunit is a domain of seven-transmembrane helixes, composed of a large extracellular domain - venus flytrap (VFT). | Each subunit is a domain of seven-transmembrane helixes, composed of a large extracellular domain - venus flytrap (VFT). | ||
| - | + | VFT contains two lobe-shaped domains: LB1 and LB2, which are connected by three short loops. | |
LB1 and LB2 are <scene name='70/701448/Gbr2_subunit/1'>αβ-folds composed of central β sheet flanked by an α helix</scene><ref>PMID:24305054</ref>. | LB1 and LB2 are <scene name='70/701448/Gbr2_subunit/1'>αβ-folds composed of central β sheet flanked by an α helix</scene><ref>PMID:24305054</ref>. | ||
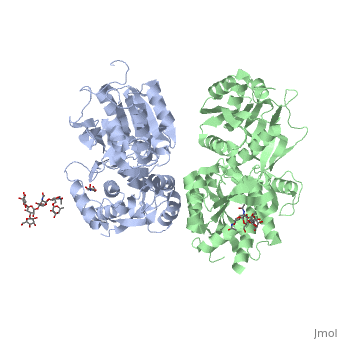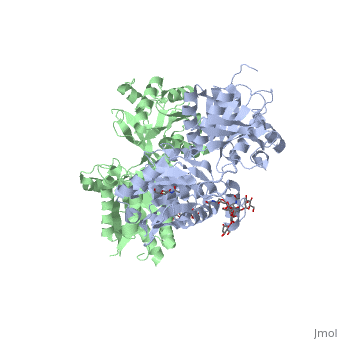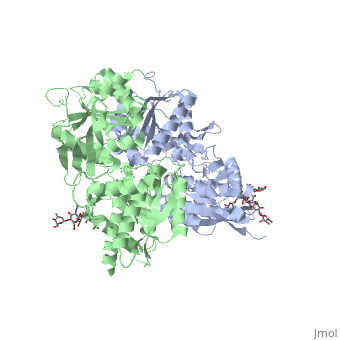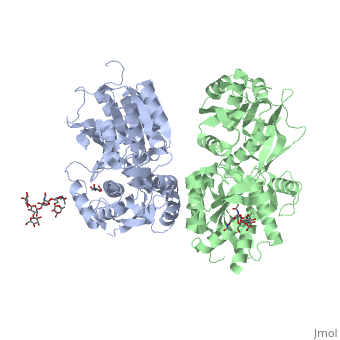
<Structure load='4mqe' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='4mqe' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
Revision as of 07:52, 18 May 2015
Contents |
Introduction
γ-Aminobutyric acid (GABA)
GABA is the major inhibitory neurotransmitter in the central nervous system (CNS). It plays a key role in modulating neuronal activity since it binds to specific transmembrane receptors (GABAA,GABAB and GABAC) in the plasma membrane of both pre- and postsynaptic neuronal level.
GABAB receptors
Mammalian GABAB receptor is a class C G-protein coupled receptor[1]. Its structure is similar to mGluR ligand binding domain. GABAB is central to inhibitory neurotransmission in the brain and so is considered a good candidate for treatments against alcoholism, stress and number of brain diseases[2].
Function
The GABAB receptor causes the opening of the K+ channels in the postsynaptic membrane, bringing the neuron closer to the equilibrium potential of K+, producing hyperpolarization. As a result the Ca+2 channels in the presynaptic terminal close and neurotransmitter release stops. GABAB can also reduce the activity of adenylyl cyclase and decrease the cell’s conductance to Ca+2.[1].
structure
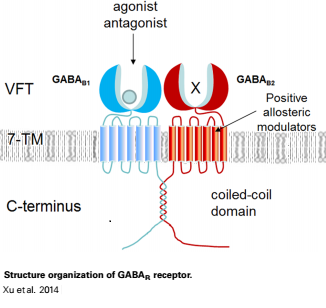
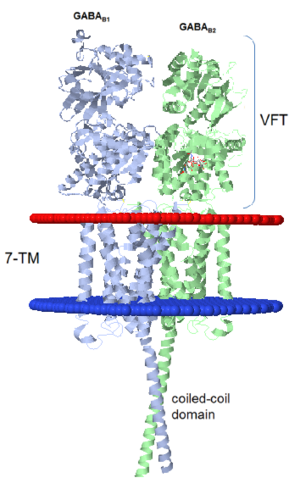
GABAB functions as an obligatory heterodimer subunit of GABAB1 (GBR1) and GABAB2 (GBR2). GBR1 is responsible for ligand-binding. GBR2, on the other hand, is responsible for G protein coupling subunits. The GABAB receptor is one of only a few obligate receptor heterodimers currently known.
GBR1 and GBR2 subunits structure
Each subunit is a domain of seven-transmembrane helixes, composed of a large extracellular domain - venus flytrap (VFT). VFT contains two lobe-shaped domains: LB1 and LB2, which are connected by three short loops. LB1 and LB2 are [3].
|
VFT interaction
The subunit association is exclusively facilitated by LB1-LB1 contact, which is mediated by the B and C helices of both of the subunits. There interface area is divided into three regions: Site I, II and III. Site I is comprised of a central hydrophobic patch surrounded by hydrogen bonds. It features three deeply buried tyrosine residues (Y113 and Y117 of GBR1bVFT, and Y118 of GBR2VFT) that are critical for heterodimer interaction and receptor activation. They are responsible for the majority of hydrophobic contacts at the LB1-LB1 heterodimer interface.
Site II interactions are mostly hydrogen bonds and include a universal salt bridge (GBR1bVFT-R141: GBR2VFT-D109) and hydrogen bond (GBR1bVFTE138: GBR2VFT-N110).
Site III consists predominantly of water-mediated contacts. It is the most variable part of the LB1-LB1 interface.
It was also shown that LB2-LB2 interface, which contains F and G helices, f and g strands, and connecting loops, is important for the receptors function. Mutations in the GBR1bVFT residues of T198, E201 and S225, and mutations in the GBR2VFT residues of D204, Q206, N213 and S233 strongly reduce signaling via G protein.
Agonist and antagonist binding
All of the agonists and antagonists bind the extracellular VFT module situated at the crevice between the LB1 and LB2 domains of the GBR1bVFT subunit.
Agonist-induced heterodimer interface
The objective of the GABAb agonist such as and , is to stabilize the closed conformation of GBR1bVFT.
Elements involved in heterodimer formation LB1-LB1: B and C helices. And the elements involved in heterodimer formation LB2-LB2: F and G helices, f and g strands, and connecting loops. Specific contacts at the LB2-LB2 heterodimer interface are divided into three regions: IV, V, and VI (hydrogen bond regions).
Antgonist-induced heterodimer interface
The objective of the GABAb antgonist like , , , and , is to confine the GBR1bVFT subunit to the open configuration of GBR1bVFT.
Dimerization motif
When the GABAb GBR1 subunit is expressed alone, it is trapped in vesicles within the cell, whereas the GABAb GBR2 alone is expressed on the cell surface, but cannot bind GABA or activate G proteins. When both receptor subunits are expressed in the same cell, the receptors interact through [4][5] . in their carboxyl tails. They are then expressed on the cell surface, bind GABA and activate G proteins. This domain is shaped by polar interactions within the and is stabilized by interactions.
Mutagenesis studies
studies of GBR1 and GBR2
Mutagenesis studies in GBR1 subunit [2]
Trp182Ala, Trp395Ala, : Abolishes signaling via G-proteins. Abolishes antagonist binding.
Tyr230Ala: Slightly decreases signaling via G-proteins.
Tyr234Ala: Decreases signaling via G-proteins.
His287Ala: Strongly reduces signaling via G-proteins. Abolishes antagonist binding.
Tyr367Ala: Strongly reduces signaling via G-proteins. No effect on antagonist binding.
Mutagenesis studies in GBR2 subunit [3]
Tyr118Ala: Impairs interaction with GABBR1. Decreases signaling via G-proteins.
References
- ↑ Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, Rosen H, Wuthrich K. The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nat Rev Drug Discov. 2013 Jan;12(1):25-34. doi: 10.1038/nrd3859. Epub 2012 Dec, 14. PMID:23237917 doi:http://dx.doi.org/10.1038/nrd3859
- ↑ Addolorato G, Leggio L, Cardone S, Ferrulli A, Gasbarrini G. Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol. 2009 Nov;43(7):559-63. doi: 10.1016/j.alcohol.2009.09.031. PMID:19913201 doi:http://dx.doi.org/10.1016/j.alcohol.2009.09.031
- ↑ Geng Y, Bush M, Mosyak L, Wang F, Fan QR. Structural mechanism of ligand activation in human GABA(B) receptor. Nature. 2013 Dec 12;504(7479):254-9. doi: 10.1038/nature12725. Epub 2013 Dec 4. PMID:24305054 doi:http://dx.doi.org/10.1038/nature12725
- ↑ Burmakina S, Geng Y, Chen Y, Fan QR. Heterodimeric coiled-coil interactions of human GABAB receptor. Proc Natl Acad Sci U S A. 2014 Apr 28. PMID:24778228 doi:http://dx.doi.org/10.1073/pnas.1400081111
- ↑ Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002 Sep;3(9):639-50. PMID:12209124 doi:http://dx.doi.org/10.1038/nrm908