We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Connexin
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
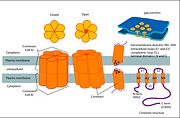
GJB2 is a gene which encodes a member of the gap junction protein family. Intercellular signaling is one of the most essential properties of multicellular organisms. [http://www.uniprot.org/uniprot/P29033 Gap junctions] are specialized membrane regions containing hundreds of intercellular communication channels that allow the passage of molecules such as ions, metabolites, nucleotides and small peptides. The gap junctions were first characterized by electron microscopy as regionally specialized structures on plasma membranes of contacting adherent cells. These structures were shown to consist of cell-to-cell channels that facilitate the transfer of ions and small molecules between cells. The gap junction proteins, also known as connexins, purified from fractions of enriched gap junctions from different tissues differ. The gap junction proteins are divided into two categories, alpha and beta. Mutations in this gene are responsible for as much as 50% of pre-lingual, recessive deafness.<ref name='Structure'>pmid 19622859</ref> | GJB2 is a gene which encodes a member of the gap junction protein family. Intercellular signaling is one of the most essential properties of multicellular organisms. [http://www.uniprot.org/uniprot/P29033 Gap junctions] are specialized membrane regions containing hundreds of intercellular communication channels that allow the passage of molecules such as ions, metabolites, nucleotides and small peptides. The gap junctions were first characterized by electron microscopy as regionally specialized structures on plasma membranes of contacting adherent cells. These structures were shown to consist of cell-to-cell channels that facilitate the transfer of ions and small molecules between cells. The gap junction proteins, also known as connexins, purified from fractions of enriched gap junctions from different tissues differ. The gap junction proteins are divided into two categories, alpha and beta. Mutations in this gene are responsible for as much as 50% of pre-lingual, recessive deafness.<ref name='Structure'>pmid 19622859</ref> | ||
| - | [[image:co.jpg| | + | [[image:co.jpg | thumb]] <ref>http://en.wikipedia.org/wiki/Connexin</ref> |
==Phenotypic results of mutations in connexin 26?== | ==Phenotypic results of mutations in connexin 26?== | ||
Mutations in human Connexin26 (hCx26) can lead to [http://www.asha.org/public/hearing/Congenital-Hearing-Loss/ congenital hearing loss] (1 child per 1000 frequency) that can be syndromic or non-syndromic. Non-syndromic hearing loss [http://omim.org/entry/603629?search=nshl&highlight=nshl (NSHL)] is characterized by sensorineural hearing loss in the absence of other symptoms, while syndromic hearing loss affects other organ systems, primarily the skin. mutations in GJB2 (the gene that encodes for Cx26) account for about half of all congenital and autosomal recessive nonsyndromic hearing loss in every population tested . Although the most frequently occurring [http://omim.org/entry/603629?search=nshl&highlight=nshl (NSHL)] mutations produce severely truncated proteins due to frameshift or missense, almost 80% of the known deafness mutations are actually single amino acid changes or deletions. These mutations have been found across the entire sequence of Cx26. The majority of NSHL mutations cause either generalized folding problems that result in the failure of Cx26 to traffic to the cell surface, or are permissive for the formation of gap junction plaques, but prevent intercellular channel function.<ref name='mutant int'>pmid 23967136</ref> | Mutations in human Connexin26 (hCx26) can lead to [http://www.asha.org/public/hearing/Congenital-Hearing-Loss/ congenital hearing loss] (1 child per 1000 frequency) that can be syndromic or non-syndromic. Non-syndromic hearing loss [http://omim.org/entry/603629?search=nshl&highlight=nshl (NSHL)] is characterized by sensorineural hearing loss in the absence of other symptoms, while syndromic hearing loss affects other organ systems, primarily the skin. mutations in GJB2 (the gene that encodes for Cx26) account for about half of all congenital and autosomal recessive nonsyndromic hearing loss in every population tested . Although the most frequently occurring [http://omim.org/entry/603629?search=nshl&highlight=nshl (NSHL)] mutations produce severely truncated proteins due to frameshift or missense, almost 80% of the known deafness mutations are actually single amino acid changes or deletions. These mutations have been found across the entire sequence of Cx26. The majority of NSHL mutations cause either generalized folding problems that result in the failure of Cx26 to traffic to the cell surface, or are permissive for the formation of gap junction plaques, but prevent intercellular channel function.<ref name='mutant int'>pmid 23967136</ref> | ||
| Line 12: | Line 12: | ||
==Connexin structure== | ==Connexin structure== | ||
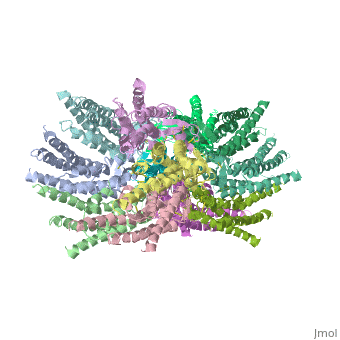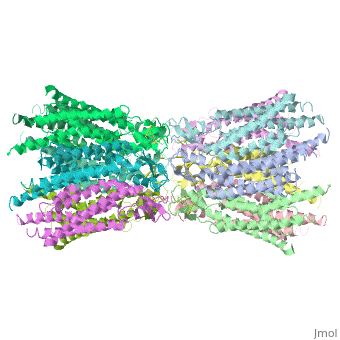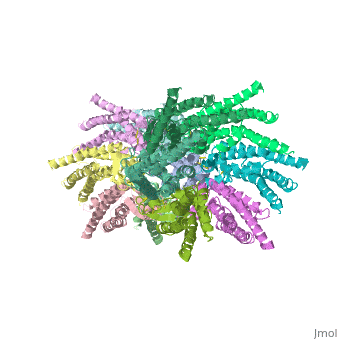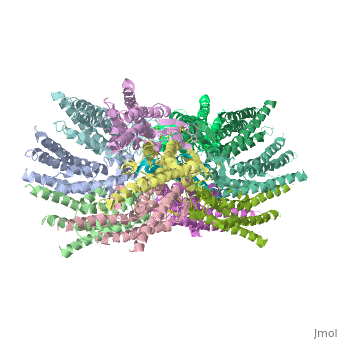
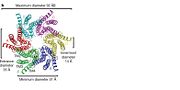
<scene name='70/701426/Connexons_secondary_structure/1'>connexins</scene> are integral α-hellical transmembrane proteins that form intercellular channels in vertebrates . Six connexins form a hexamerical assembly, known as <scene name='70/701426/Connexin_26_basic_structure/2'>Connexon</scene> or hemichannel , which delineates an aqueous pore with a minimum diameter of ∼1.2 nm. When two hemichannels from adjacent cells dock and join, leaving a gap of ∼2–3 nm, they may form an intercellular [http://www.uniprot.org/uniprot/P29033 gap junction channel] which spans the two plasma membranes and allows the exchange of cytoplasmic molecules with size up to ∼1 kDa. | <scene name='70/701426/Connexons_secondary_structure/1'>connexins</scene> are integral α-hellical transmembrane proteins that form intercellular channels in vertebrates . Six connexins form a hexamerical assembly, known as <scene name='70/701426/Connexin_26_basic_structure/2'>Connexon</scene> or hemichannel , which delineates an aqueous pore with a minimum diameter of ∼1.2 nm. When two hemichannels from adjacent cells dock and join, leaving a gap of ∼2–3 nm, they may form an intercellular [http://www.uniprot.org/uniprot/P29033 gap junction channel] which spans the two plasma membranes and allows the exchange of cytoplasmic molecules with size up to ∼1 kDa. | ||
| - | The height of the modelled structure of the gap junction channel without disordered cytoplasmic loop and C-terminal segment is approximately 155Å. The transmembrane region and membrane surfaces were deduced from the distribution of hydrophobic and aromatic amino acid residues along the noncrystallographic six-fold axis It is a tsuzumi shape, a traditional Japanese drum.<ref name='Structure'/> [[Image:distances a.jpg| | + | The height of the modelled structure of the gap junction channel without disordered cytoplasmic loop and C-terminal segment is approximately 155Å. The transmembrane region and membrane surfaces were deduced from the distribution of hydrophobic and aromatic amino acid residues along the noncrystallographic six-fold axis It is a tsuzumi shape, a traditional Japanese drum.<ref name='Structure'/> [[Image:distances a.jpg | thumb]] |
The protomers in each hexameric connexon are related by a sixfold non-crystallographic symmetry (NCS) axis perpendicular to the membrane plane . The transmembrane region of the channel is 38Å thick.TM2 extends about 19Å from the membrane surface into the cytoplasm. The extracellular region of the connexon extends 23Å from the membrane surface and interdigitates to the opposite connexon by 6Å, resulting in the intercellular ‘gap’ of 40Å. The extracellular lobes are not protruding so much, as indicated by the structural analyses of split gap junction channels with atomic force microscopy and electron microscopy. The relatively flat lobes could be attributed to the conformational change of the extracellular region induced by the docking of two connexons. The diameter of the connexon is biggest at the cytoplasmic side of the membrane, 92Å , and smallest at the extracellular side, 51Å . | The protomers in each hexameric connexon are related by a sixfold non-crystallographic symmetry (NCS) axis perpendicular to the membrane plane . The transmembrane region of the channel is 38Å thick.TM2 extends about 19Å from the membrane surface into the cytoplasm. The extracellular region of the connexon extends 23Å from the membrane surface and interdigitates to the opposite connexon by 6Å, resulting in the intercellular ‘gap’ of 40Å. The extracellular lobes are not protruding so much, as indicated by the structural analyses of split gap junction channels with atomic force microscopy and electron microscopy. The relatively flat lobes could be attributed to the conformational change of the extracellular region induced by the docking of two connexons. The diameter of the connexon is biggest at the cytoplasmic side of the membrane, 92Å , and smallest at the extracellular side, 51Å . | ||
Viewed from the top, the channel looks like a ‘hexagonal nut’ with a pore in the centre .The diameter of the pore is about 40Å at the cytoplasmic side of the channel, narrowing to 14Å near the extracellular membrane surface and then widening to 25Å in the extracellular space.<ref name='Structure'/> | Viewed from the top, the channel looks like a ‘hexagonal nut’ with a pore in the centre .The diameter of the pore is about 40Å at the cytoplasmic side of the channel, narrowing to 14Å near the extracellular membrane surface and then widening to 25Å in the extracellular space.<ref name='Structure'/> | ||
| - | [[Image:distances b.jpg| | + | [[Image:distances b.jpg | thumb]] |
==Structure of the cx26 protomer:== | ==Structure of the cx26 protomer:== | ||
The [http://en.wikipedia.org/wiki/Promoter_(genetics) protomer] has four transmembrane segments (TM1–4), two extracellular loops (E1 and E2), a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment (Fig. 3). Cx26 forms a typical four-helix bundle in which any pair of adjacent helices is antiparallel. TM1 and TM2 face the interior, whereas TM3 and TM4 face the hydrophobic membrane environment. There has been controversy about the identity of the major pore-lining helix, on the basis of accessibility studies of substituted cysteines and sequence analysis. The major pore-lining helix TM1 is inclined, so that the pore diameter narrows from the cytoplasmic to the extracellular side of the membrane, and ends in a short 310 helix. | The [http://en.wikipedia.org/wiki/Promoter_(genetics) protomer] has four transmembrane segments (TM1–4), two extracellular loops (E1 and E2), a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment (Fig. 3). Cx26 forms a typical four-helix bundle in which any pair of adjacent helices is antiparallel. TM1 and TM2 face the interior, whereas TM3 and TM4 face the hydrophobic membrane environment. There has been controversy about the identity of the major pore-lining helix, on the basis of accessibility studies of substituted cysteines and sequence analysis. The major pore-lining helix TM1 is inclined, so that the pore diameter narrows from the cytoplasmic to the extracellular side of the membrane, and ends in a short 310 helix. | ||
Revision as of 10:43, 18 May 2015
| |||||||||||
References
- ↑ Zonta F, Buratto D, Cassini C, Bortolozzi M, Mammano F. Molecular dynamics simulations highlight structural and functional alterations in deafness-related M34T mutation of connexin 26. Front Physiol. 2014 Mar 4;5:85. doi: 10.3389/fphys.2014.00085. eCollection 2014. PMID:24624091 doi:http://dx.doi.org/10.3389/fphys.2014.00085
- ↑ 2.0 2.1 2.2 2.3 2.4 Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009 Aug;65(Pt 8):758-66. Epub 2009, Jul 10. PMID:19622859 doi:http://dx.doi.org/10.1107/S0907444909014711
- ↑ http://en.wikipedia.org/wiki/Connexin
- ↑ 4.0 4.1 Ambrosi C, Walker AE, Depriest AD, Cone AC, Lu C, Badger J, Skerrett IM, Sosinsky GE. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 2013 Aug 15;8(8):e70916. doi: 10.1371/journal.pone.0070916. eCollection, 2013. PMID:23967136 doi:http://dx.doi.org/10.1371/journal.pone.0070916
- ↑ 5.0 5.1 5.2 Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric Configurations and N-terminal Rearrangements in Connexin26 Gap Junction Channels. J Mol Biol. 2011 Jan 21;405(3):724-35. Epub 2010 Nov 20. PMID:21094651 doi:10.1016/j.jmb.2010.10.032
Proteopedia Page Contributors and Editors (what is this?)
Safaa Salah Hussiesy, Michal Harel, Doaa Naffaa, Jaime Prilusky