We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
PLC beta 3 Gq
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
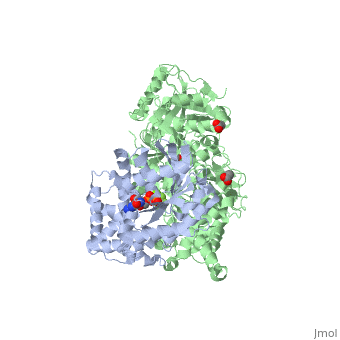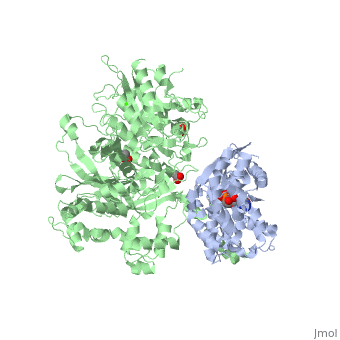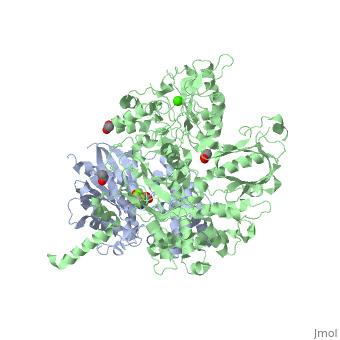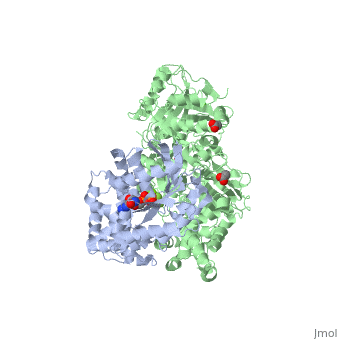
The canonical Gα effector-binding region of Gαq, located between α3 and switch 2, is occupied by a helix-turn-helix (Hα1/Hα2) that immediately follows the C2 domain of PLC- β3. <scene name='70/701452/Pro862/2'>Pro862</scene> of PLC- β3 lies within the turn between Hα1 and Hα2, makes extensive contacts with multiple residues of Gαq, and forms the center of a Gαq-binding interface. | The canonical Gα effector-binding region of Gαq, located between α3 and switch 2, is occupied by a helix-turn-helix (Hα1/Hα2) that immediately follows the C2 domain of PLC- β3. <scene name='70/701452/Pro862/2'>Pro862</scene> of PLC- β3 lies within the turn between Hα1 and Hα2, makes extensive contacts with multiple residues of Gαq, and forms the center of a Gαq-binding interface. | ||
| - | <scene name='70/701452/Fig6/5'> | + | <scene name='70/701452/Fig6/5'>The loop</scene> between the end of the TIM barrel and the beginning of the C2 domain comprises a second distinct segment of PLC- β3 that makes extensive contacts with active Gαq, including switches 1 and 2. This interface includes a series of interdigitated pairs of charged residues, specifically in PLC- β3-Gαq Asp709/Arg202, Lys710/Glu191, and Asp721/Lys41; these in turn are supported by additional charged residues Glu703 and Arg707 of PLC- β3. |
<scene name='70/701452/Fig7/1'>An extended loop</scene> between EF hands 3 and 4 of PLC- β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | <scene name='70/701452/Fig7/1'>An extended loop</scene> between EF hands 3 and 4 of PLC- β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | ||
Revision as of 14:39, 18 May 2015
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
| |||||||||||
References
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403