We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Connexin
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
=Structure:= | =Structure:= | ||
==Connexin structure== | ==Connexin structure== | ||
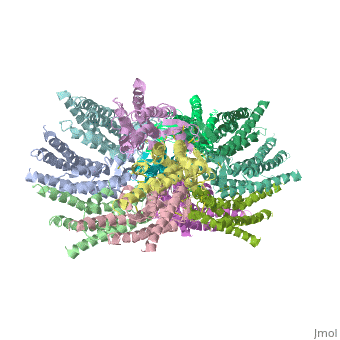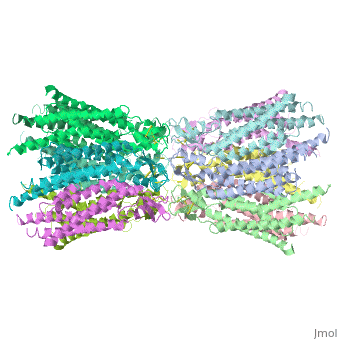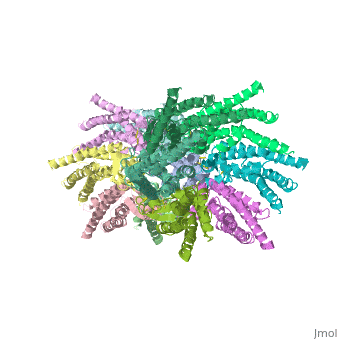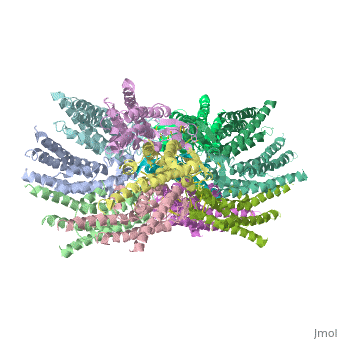
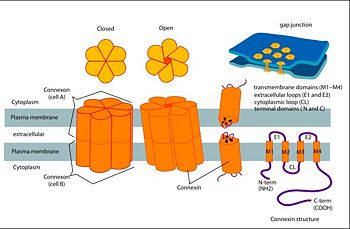
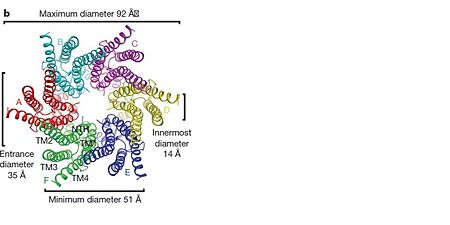
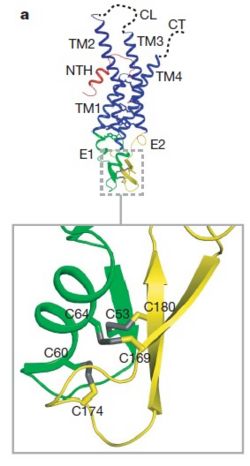
| - | Connexins are integral transmembranal proteins which consist of α-helical domains in the <scene name='70/701426/Connexin_26_basic_structure/6'>4 transmembrane segments</scene> (TM1-TM4) , <scene name='70/701426/two extracellular loops/1'>two extracellular segments</scene> (E1 and E2), a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment.<scene name='70/701426/Connexons_secondary_structure/1'>This scene</scene> shows the overall connexon secondary structure. The overall contents of a single unit of a connexin is shown in <scene name='70/701426/Connexin_structure2/1'>this scene</scene>. Each connexin consists of 227 amino acids from the <scene name='70/701426/Connexin_26_basic_structure/3'>N terminus to the C terminus</scene>. Connexins form intercellular channels in vertebrates . Six connexins form a hexamerical assembly, known as <scene name='70/701426/Connexin_26_basic_structure/5'>a connexxon or a hemmichannel</scene> , which delineates an aqueous pore with a minimum diameter of ∼1.2 nm. When two hemichannels from adjacent cells dock and join, leaving a gap of ∼2–3 nm, they may form an intercellular [http://www.uniprot.org/uniprot/P29033 gap junction channel] which spans the two plasma membranes and allows the exchange of cytoplasmic molecules with size up to ∼1 kDa. | + | <scene name='70/701426/Wild_type_connexin/1'>Connexins</scene> are integral transmembranal proteins which consist of α-helical domains in the <scene name='70/701426/Connexin_26_basic_structure/6'>4 transmembrane segments</scene> (TM1-TM4) , <scene name='70/701426/two extracellular loops/1'>two extracellular segments</scene> (E1 and E2), a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment.<scene name='70/701426/Connexons_secondary_structure/1'>This scene</scene> shows the overall connexon secondary structure. The overall contents of a single unit of a connexin is shown in <scene name='70/701426/Connexin_structure2/1'>this scene</scene>. Each connexin consists of 227 amino acids from the <scene name='70/701426/Connexin_26_basic_structure/3'>N terminus to the C terminus</scene>. Connexins form intercellular channels in vertebrates . Six connexins form a hexamerical assembly, known as <scene name='70/701426/Connexin_26_basic_structure/5'>a connexxon or a hemmichannel</scene> , which delineates an aqueous pore with a minimum diameter of ∼1.2 nm. When two hemichannels from adjacent cells dock and join, leaving a gap of ∼2–3 nm, they may form an intercellular [http://www.uniprot.org/uniprot/P29033 gap junction channel] which spans the two plasma membranes and allows the exchange of cytoplasmic molecules with size up to ∼1 kDa. |
The height of the modelled structure of the gap junction channel without disordered cytoplasmic loop and C-terminal segment is approximately 155Å.<ref name='Structure'/> [[Image:distances a.jpg | thumb | 350px |center]] | The height of the modelled structure of the gap junction channel without disordered cytoplasmic loop and C-terminal segment is approximately 155Å.<ref name='Structure'/> [[Image:distances a.jpg | thumb | 350px |center]] | ||
| Line 31: | Line 31: | ||
In general, single site mutations are spread fairly evenly across the whole protein with TM2 having the highest mutation density (number of amino acids with NHLS mutations divided by the total number of amino acids in the domain) at 67% to M1 and E1 having the lowest density of mutations with their respective domains at 33%. According to this criterion, TM4 has a mutation density of 40%. . Of the four transmembrane helices, M1, M2 and M3 have attracted the most attention, because of the controversies involved in models with different helix assignments, based on lower resolution cryo-electron crystallographic structures and scanning cysteine accessibility mutagenesis . Far less is known about TM4 and how side chains interact with the other helices and with the lipid bilayer. <ref name='mutant int'/> | In general, single site mutations are spread fairly evenly across the whole protein with TM2 having the highest mutation density (number of amino acids with NHLS mutations divided by the total number of amino acids in the domain) at 67% to M1 and E1 having the lowest density of mutations with their respective domains at 33%. According to this criterion, TM4 has a mutation density of 40%. . Of the four transmembrane helices, M1, M2 and M3 have attracted the most attention, because of the controversies involved in models with different helix assignments, based on lower resolution cryo-electron crystallographic structures and scanning cysteine accessibility mutagenesis . Far less is known about TM4 and how side chains interact with the other helices and with the lipid bilayer. <ref name='mutant int'/> | ||
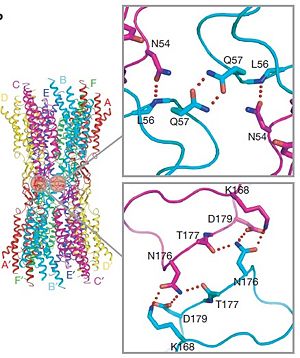
| - | Electron crystallographic studies yielded a three-dimensional (3D) structure of a C-terminal truncated connexin43 gap junction channel, with each half containing 24 α-helices arranged with a 6-fold symmetry. The 3D structure of a mutant human connexin26 (Cx26M34A) channel shows an unexpected density within the vestibule of each hemichannel, which is called a "plug". Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'>pmid 21094651</ref>, two 3D structures of the Cx26M34A gap junctions are available, the first is the <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>6-Å resolution structure of | + | Electron crystallographic studies yielded a three-dimensional (3D) structure of a C-terminal truncated connexin43 gap junction channel, with each half containing 24 α-helices arranged with a 6-fold symmetry. The 3D structure of a mutant human connexin26 (Cx26M34A) channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene>, which is called a "plug". Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'>pmid 21094651</ref>, two 3D structures of the Cx26M34A gap junctions are available, the first is the <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>6-Å resolution structure of |
Cx26M34A channels</scene> <ref name='pdb'/> and the second is the <scene name='70/701426/Deletion_of_cx26m34adel2-7/1'>The N-terminal deletion of Cx26M34A4adel2-7</scene> <ref name='pdb'/> in which | Cx26M34A channels</scene> <ref name='pdb'/> and the second is the <scene name='70/701426/Deletion_of_cx26m34adel2-7/1'>The N-terminal deletion of Cx26M34A4adel2-7</scene> <ref name='pdb'/> in which | ||
amino acids 2–7 were deleted. | amino acids 2–7 were deleted. | ||
Revision as of 15:22, 19 May 2015
| |||||||||||
References
- ↑ Zonta F, Buratto D, Cassini C, Bortolozzi M, Mammano F. Molecular dynamics simulations highlight structural and functional alterations in deafness-related M34T mutation of connexin 26. Front Physiol. 2014 Mar 4;5:85. doi: 10.3389/fphys.2014.00085. eCollection 2014. PMID:24624091 doi:http://dx.doi.org/10.3389/fphys.2014.00085
- ↑ 2.0 2.1 2.2 2.3 Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009 Aug;65(Pt 8):758-66. Epub 2009, Jul 10. PMID:19622859 doi:http://dx.doi.org/10.1107/S0907444909014711
- ↑ http://en.wikipedia.org/wiki/Connexin
- ↑ 4.0 4.1 Ambrosi C, Walker AE, Depriest AD, Cone AC, Lu C, Badger J, Skerrett IM, Sosinsky GE. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 2013 Aug 15;8(8):e70916. doi: 10.1371/journal.pone.0070916. eCollection, 2013. PMID:23967136 doi:http://dx.doi.org/10.1371/journal.pone.0070916
- ↑ 5.0 5.1 5.2 Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric Configurations and N-terminal Rearrangements in Connexin26 Gap Junction Channels. J Mol Biol. 2011 Jan 21;405(3):724-35. Epub 2010 Nov 20. PMID:21094651 doi:10.1016/j.jmb.2010.10.032
Proteopedia Page Contributors and Editors (what is this?)
Safaa Salah Hussiesy, Michal Harel, Doaa Naffaa, Jaime Prilusky