Regulator of G protein signaling
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
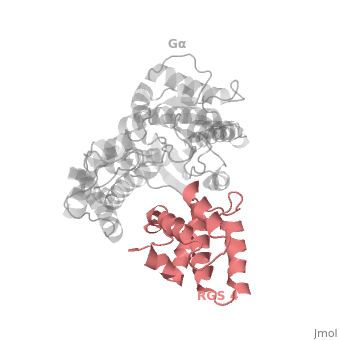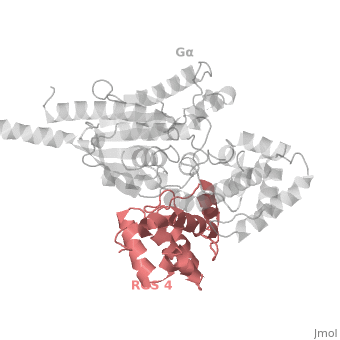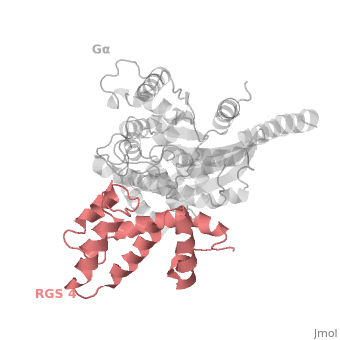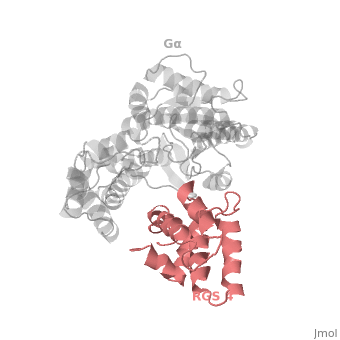
Gα<sub>i1</sub> subunits adopt a conserved fold composed of <scene name='70/701447/All-helical-domain/6'>α helical domain</scene> , a helical domain of six α helices shown as blue cartoon and a GTPase domain shown in gray cartoons. The GTPase domain hydrolyzes GTP and provides most of Gα's binding surfaces for Gβγ, receptors, effectors and RGS proteins. <scene name='70/701447/Gi-rgs4/20'>The GTPase domain</scene> contains three flexible regions designated switch-I presented as blue sticks, switch-II presented as magenta sticks and switch-III presented as green sticks that change conformation in response to GTP binding and hydrolysis, GDP–Mg<sup>+2</sup>, bound in the active site of Gα<sub>i1</sub> is shown as a ball-and-stick model. The three switch regions of Gα<sub>i1</sub>: residues 176–184, 201–215, and 233–241, respectively . <ref>PMID: 9108480</ref> | Gα<sub>i1</sub> subunits adopt a conserved fold composed of <scene name='70/701447/All-helical-domain/6'>α helical domain</scene> , a helical domain of six α helices shown as blue cartoon and a GTPase domain shown in gray cartoons. The GTPase domain hydrolyzes GTP and provides most of Gα's binding surfaces for Gβγ, receptors, effectors and RGS proteins. <scene name='70/701447/Gi-rgs4/20'>The GTPase domain</scene> contains three flexible regions designated switch-I presented as blue sticks, switch-II presented as magenta sticks and switch-III presented as green sticks that change conformation in response to GTP binding and hydrolysis, GDP–Mg<sup>+2</sup>, bound in the active site of Gα<sub>i1</sub> is shown as a ball-and-stick model. The three switch regions of Gα<sub>i1</sub>: residues 176–184, 201–215, and 233–241, respectively . <ref>PMID: 9108480</ref> | ||
| - | == RGS-G proteins | + | == RGS-G proteins interactions == |
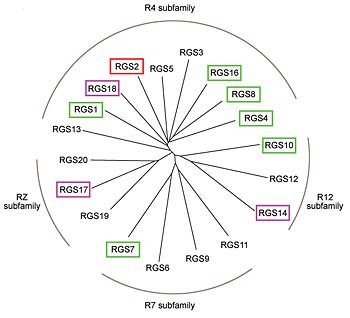
There are many RGS protein residues in the vicinity of the RGS domain–Gα interface that contribute to RGS-G proteins interaction. These residues classified into two major groups. First group is Significant & Conserved residues that located mainly in the center of the RGS domain–Gα interface and have the primary role in accelerating Gα GTPase by stabilizing Gα in a conformation optimal for GTP hydrolysis. Whereas the second group is putative Modulatory residues that located mostly at the periphery of this interface where they contribute to Gα subunit recognition.<ref>PMID: 21685921</ref> | There are many RGS protein residues in the vicinity of the RGS domain–Gα interface that contribute to RGS-G proteins interaction. These residues classified into two major groups. First group is Significant & Conserved residues that located mainly in the center of the RGS domain–Gα interface and have the primary role in accelerating Gα GTPase by stabilizing Gα in a conformation optimal for GTP hydrolysis. Whereas the second group is putative Modulatory residues that located mostly at the periphery of this interface where they contribute to Gα subunit recognition.<ref>PMID: 21685921</ref> | ||
Revision as of 12:08, 23 May 2015
Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gαi as a model structure.
| |||||||||||
References
- ↑ Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
- ↑ Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147 Suppl 1:S46-55. PMID:16402120 doi:http://dx.doi.org/10.1038/sj.bjp.0706405
- ↑ Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997 Apr 18;89(2):251-61. PMID:9108480
- ↑ Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
Proteopedia Page Contributors and Editors (what is this?)
Ali Asli, Denise Salem, Michal Harel, Joel L. Sussman, Jaime Prilusky