Carnitine acetyltransferase
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
* '''The role of carnitine acyltransferases in fatty acid oxidation''' | * '''The role of carnitine acyltransferases in fatty acid oxidation''' | ||
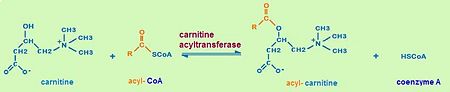
The most important biological function of carnitine acyltransferases is the transport of fatty acids for β-oxidation<ref name="structure" />.Fatty acids are oxidized for energy production in the mitochondrial matrix by a process called β- oxidation. The major site of fatty acid accumulation, however, is the cytoplasm of the cells. Hence, in order to provide energy, fatty acids have to be transported from the cytoplasm across the inner mitochondrial membrane into the mitochondrial matrix. The carnitine shuttle, a transport chain that consists of three enzymatic reactions, helps fatty acids to pass the mitochondrial membrane. Carnitine acyltransferases (CrATs, COTs, CPTs) are part of the carnitine shuttle. | The most important biological function of carnitine acyltransferases is the transport of fatty acids for β-oxidation<ref name="structure" />.Fatty acids are oxidized for energy production in the mitochondrial matrix by a process called β- oxidation. The major site of fatty acid accumulation, however, is the cytoplasm of the cells. Hence, in order to provide energy, fatty acids have to be transported from the cytoplasm across the inner mitochondrial membrane into the mitochondrial matrix. The carnitine shuttle, a transport chain that consists of three enzymatic reactions, helps fatty acids to pass the mitochondrial membrane. Carnitine acyltransferases (CrATs, COTs, CPTs) are part of the carnitine shuttle. | ||
| - | [[Image:Second step carnitine shuttle.jpg|thumb|second step of the carnitine shuttle| | + | [[Image:Second step carnitine shuttle.jpg|thumb|second step of the carnitine shuttle|450px|left]] |
The first step of the carnitine shuttle is the activation of fatty acids. They are transformed into the activated form (= acyl CoA) by the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A. The '''second step''' of the carnitine shuttle is catalyzed by <font color='#802828'>'''carnitine acyltransferases'''</font>. These enzymes (especially CPT) facilitate the transport of fatty acids by conjugating them to <font color='#267DAB'>'''carnitine'''</font>. In this reaction an <font color='#c88033'>'''acyl group'''</font> is transferred from the sulfur atom of <font color='#00007C'>'''CoA'''</font> to the hydroxyl group of carnitine. The product is <font color='#c88033'>'''acyl-'''</font> <font color='#267DAB'>'''carnitine'''</font>. The third step of the carnitine shuttle is also catalyzed by <font color='#802828'>'''carnitine acyltransferases'''</font> (especially CPT) in the mitochondrial matrix. In this reaction the <font color='#c88033'>'''acyl group'''</font> of <font color='#c88033'>'''acyl-'''</font> <font color='#267DAB'>'''carnitine'''</font> ester is transferred back to <font color='#00007C'>'''CoA'''</font> to form <font color='#c88033'>'''acyl-'''</font> <font color='#00007C'>'''CoA'''</font>. <ref> Lehninger Principles of Biochemistry |ISBN-10: 071677108X | ISBN-13: 978-0716771081 | Publication Date: February 1, 2008 | Edition: 5th </ref> | The first step of the carnitine shuttle is the activation of fatty acids. They are transformed into the activated form (= acyl CoA) by the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A. The '''second step''' of the carnitine shuttle is catalyzed by <font color='#802828'>'''carnitine acyltransferases'''</font>. These enzymes (especially CPT) facilitate the transport of fatty acids by conjugating them to <font color='#267DAB'>'''carnitine'''</font>. In this reaction an <font color='#c88033'>'''acyl group'''</font> is transferred from the sulfur atom of <font color='#00007C'>'''CoA'''</font> to the hydroxyl group of carnitine. The product is <font color='#c88033'>'''acyl-'''</font> <font color='#267DAB'>'''carnitine'''</font>. The third step of the carnitine shuttle is also catalyzed by <font color='#802828'>'''carnitine acyltransferases'''</font> (especially CPT) in the mitochondrial matrix. In this reaction the <font color='#c88033'>'''acyl group'''</font> of <font color='#c88033'>'''acyl-'''</font> <font color='#267DAB'>'''carnitine'''</font> ester is transferred back to <font color='#00007C'>'''CoA'''</font> to form <font color='#c88033'>'''acyl-'''</font> <font color='#00007C'>'''CoA'''</font>. <ref> Lehninger Principles of Biochemistry |ISBN-10: 071677108X | ISBN-13: 978-0716771081 | Publication Date: February 1, 2008 | Edition: 5th </ref> | ||
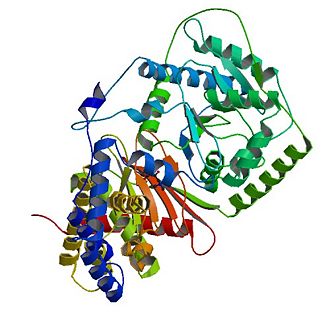
== Structure of Carnitine acetyltransferase == | == Structure of Carnitine acetyltransferase == | ||
| Line 36: | Line 36: | ||
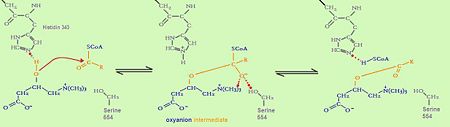
==Catalytic Mechanism of Carnitine Acyltransferases== | ==Catalytic Mechanism of Carnitine Acyltransferases== | ||
| - | [[Image:Mechanism fatty acid transfer.jpg|thumb|catalytic mechanism of fatty acid transfer| | + | [[Image:Mechanism fatty acid transfer.jpg|thumb|catalytic mechanism of fatty acid transfer|450px|left]] |
It is assumed that the whole family of carnitine acyltransferases share the same catalytic mechanism, because certain residues in the catalytic side (histidine343, serine554) are conserved throughout the family. | It is assumed that the whole family of carnitine acyltransferases share the same catalytic mechanism, because certain residues in the catalytic side (histidine343, serine554) are conserved throughout the family. | ||
Histidine 343 is probably the most important residue in catalysis. First, it induces optimal substrate binding by forming a hydrogen bond between its side chain and the hydrogen atom of the substrate’s reactive group. As soon as all substrates attained the right position, the catalytic histidine residue is ready to extract a proton from either the hydroxyl group of carnitine or the thiol group of CoA. The catalytic histidine residue can be considered as a general base in catalysis.Which proton is extracted depends on the direction of the reaction. Acyl- carnitine is formed by extracting a proton from carnitine, whereas acyl-CoA is formed by extracting a proton from CoA. | Histidine 343 is probably the most important residue in catalysis. First, it induces optimal substrate binding by forming a hydrogen bond between its side chain and the hydrogen atom of the substrate’s reactive group. As soon as all substrates attained the right position, the catalytic histidine residue is ready to extract a proton from either the hydroxyl group of carnitine or the thiol group of CoA. The catalytic histidine residue can be considered as a general base in catalysis.Which proton is extracted depends on the direction of the reaction. Acyl- carnitine is formed by extracting a proton from carnitine, whereas acyl-CoA is formed by extracting a proton from CoA. | ||
Revision as of 14:27, 27 May 2015
| |||||||||||
3D structures of carnitine acetyltransferase
Updated on 27-May-2015
1ndb – mCAT – mouse
1t7n – mCAT (mutant)
1ndf – mCAT + carnitine
1t7o – mCAT (mutant) + carnitine
2h3u – mCAT + carnitine + CoA
1t7q – mCAT (mutant) + carnitine + CoA
2h3w – mCAT (mutant) + hexanoylcarnitine + CoA
2h3p – mCAT + Carnitine + acetyl-CoA
1ndi – mCAT + CoA
1s5o – hCAT + carnitine - human
1nm8 – hCAT
References
- ↑ 1.0 1.1 Donghai Wu‡, Lakshmanan Govindasamy§, Wei Lian‡, Yunrong Gu‡, Thomas Kukar‡,Mavis Agbandje-McKenna§, and Robert McKenna§¶.Structure of Human Carnitine Acetyltransferase.Published, JBC Papers in Press, January 31, 2003 DOI 10.1074/jbc.M21235620
- ↑ 2.0 2.1 2.2 Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003 Jan 10;112(1):113-22. PMID:12526798
- ↑ Lehninger Principles of Biochemistry |ISBN-10: 071677108X | ISBN-13: 978-0716771081 | Publication Date: February 1, 2008 | Edition: 5th
- ↑ 4.0 4.1 Jogl G, Hsiao YS, Tong L. Structure and function of carnitine acyltransferases. Ann N Y Acad Sci. 2004 Nov;1033:17-29. PMID:15591000 doi:10.1196/annals.1320.002
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Alina Spielmann, Ndeye Coumba