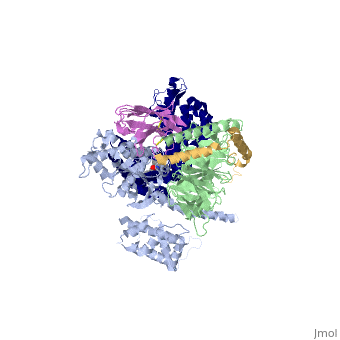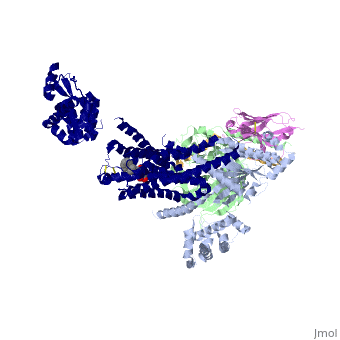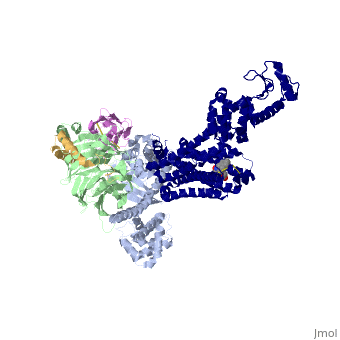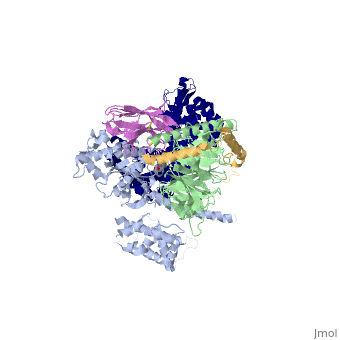
Beta2 adrenergic receptor-Gs protein complex
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
[[Image:ImgSmall1.jpg|500px|G protein cycle for the b2AR–Gs complex. Reprinted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: Nature 477, 549–555, copyright 2011]] | [[Image:ImgSmall1.jpg|500px|G protein cycle for the b2AR–Gs complex. Reprinted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: Nature 477, 549–555, copyright 2011]] | ||
| - | The figure shows the G Protein cycle <ref>doi:10.1038/nature10361</ref> - an extracellular agonist binds to the β2AR leads | + | The figure shows the G Protein cycle <ref>doi:10.1038/nature10361</ref> - an extracellular agonist binds to the β2AR leads <scene name='70/701430/Receptor_morphing_animation/1'>to conformational rearrangements of the cytoplasmic ends of transmembrane segments</scene> that enable the Gs heterotrimer to bind the receptor. GDP is released from the α subunit upon formation of β2AR–Gs complex. The GTP binds to the nucleotide-free α subunit resulting in dissociation of the α and βγ subunits from the receptor. The subunits regulate their respective effector proteins adenylyl cyclase (AC) and Ca2+ channels. The Gs heterotrimer reassembles from α and βγ subunits following hydrolysis of GTP to GDP in the α subunit. |
The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. In the nucleotide-free state, the αAH domain has a variable position relative the αRas domain. | The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. In the nucleotide-free state, the αAH domain has a variable position relative the αRas domain. | ||
Revision as of 17:10, 31 May 2015
Beta2 adrenergic receptor-Gs protein complex
| |||||||||||
References
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
Proteopedia Page Contributors and Editors (what is this?)
Dan Elran, Michal Harel, Alexander Berchansky, Joel L. Sussman