We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Beta2 adrenergic receptor-Gs protein complex
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== G-Protein-GPCR Intercations == | == G-Protein-GPCR Intercations == | ||
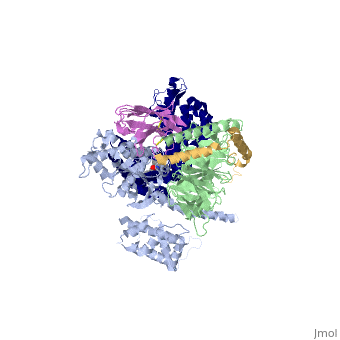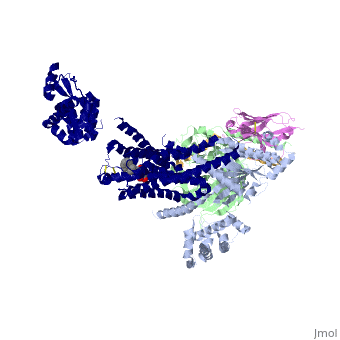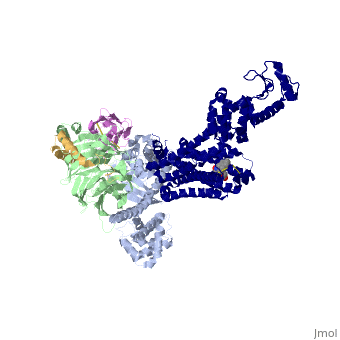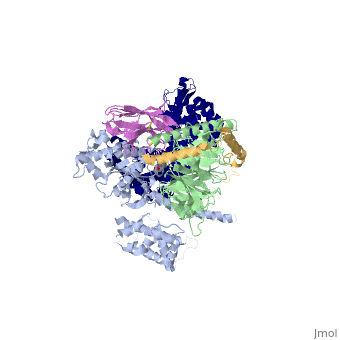
| - | The a5-helix of Gas docks into a cavity formed on the intracellular side of the receptor by the opening of transmembrane helices 5 and 6. Within the transmembrane core, the interactions are primarily non-polar. An exception involves <scene name='70/701430/Receptor_g_protein_interaction/ | + | The a5-helix of Gas docks into a cavity formed on the intracellular side of the receptor by the opening of transmembrane helices 5 and 6. Within the transmembrane core, the interactions are primarily non-polar. An exception involves <scene name='70/701430/Receptor_g_protein_interaction/3'>packing of Tyr 391 of the a5-helix against Arg 131 of the conservedDRYsequence inTM3. Arg 131 also packs against Tyr 326 of the conserved |
NPxxY sequence in TM7</scene> | NPxxY sequence in TM7</scene> | ||
Revision as of 10:20, 3 June 2015
Beta2 adrenergic receptor-Gs protein complex
| |||||||||||
References
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
Proteopedia Page Contributors and Editors (what is this?)
Dan Elran, Michal Harel, Alexander Berchansky, Joel L. Sussman