We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Beta2 adrenergic receptor-Gs protein complex
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
== G-Protein variability == | == G-Protein variability == | ||
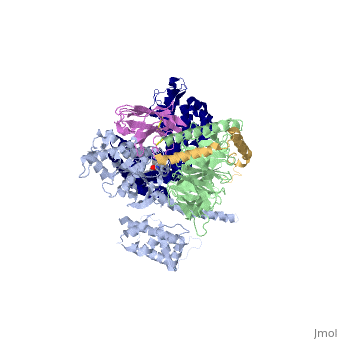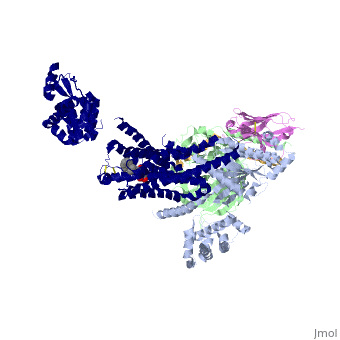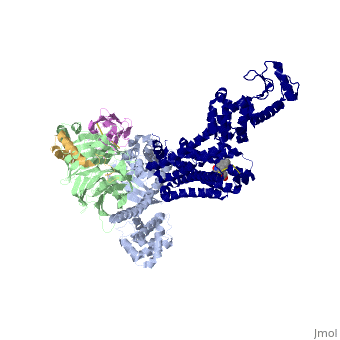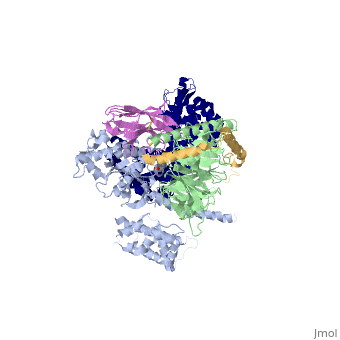
| - | The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. A big discovery made thanks to this structure is that the G alpha subunit has large variability between its GTP bound (active) and nucleotide free states, where <scene name='70/701430/Gamorph/ | + | The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. A big discovery made thanks to this structure is that the G alpha subunit has large variability between its GTP bound (active) and nucleotide free states, where <scene name='70/701430/Gamorph/2'>the αAH domain has a variable position relative to the αRas domain</scene> |
==See Also== | ==See Also== | ||
Revision as of 18:55, 3 June 2015
Beta2 adrenergic receptor-Gs protein complex
| |||||||||||
References
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
Proteopedia Page Contributors and Editors (what is this?)
Dan Elran, Michal Harel, Alexander Berchansky, Joel L. Sussman