Chymotrypsin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='7gch' size='450' side='right' scene='38/387136/Bovine_chymotrypsin_overview/1' caption=' | + | <StructureSection load='7gch' size='450' side='right' scene='38/387136/Bovine_chymotrypsin_overview/1' caption=''> |
[[Image:2ea3.png|left|200px|thumb|Crystal Structure of ''Cellulomonas Bogoriensis'' Chymotrypsin [[2ea3]]]] | [[Image:2ea3.png|left|200px|thumb|Crystal Structure of ''Cellulomonas Bogoriensis'' Chymotrypsin [[2ea3]]]] | ||
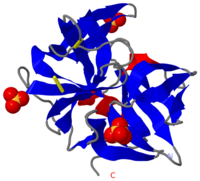
[[Chymotrypsin]] (Chy or α-Chy) is a digestive enzyme containing an active serine residue, which helps to digest proteins in our food. Other related proteases are crucial for blood clotting ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer&part=A1378&rendertype=figure&id=A1401 thrombin and other proteases]), for the AIDS virus metabolism ([http://www.proteopedia.org/wiki/index.php/Hiv_protease HIV protease]) and for many other processes relevant to human health and agriculture. Chymotrypsin cleaves peptide bonds of proteins where the amide side of the bond is an aromatic amino acid like tyrosine, phenylalanine or tryptophan. The image at the left is the crystal structure of chymotrypsin from ''Cellulomonas Bogoriensis'' ([[2ea3]]) with sulfate ions. Below is description of the structure of bovine chymotrypsin. Some additional details in<br /> | [[Chymotrypsin]] (Chy or α-Chy) is a digestive enzyme containing an active serine residue, which helps to digest proteins in our food. Other related proteases are crucial for blood clotting ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer&part=A1378&rendertype=figure&id=A1401 thrombin and other proteases]), for the AIDS virus metabolism ([http://www.proteopedia.org/wiki/index.php/Hiv_protease HIV protease]) and for many other processes relevant to human health and agriculture. Chymotrypsin cleaves peptide bonds of proteins where the amide side of the bond is an aromatic amino acid like tyrosine, phenylalanine or tryptophan. The image at the left is the crystal structure of chymotrypsin from ''Cellulomonas Bogoriensis'' ([[2ea3]]) with sulfate ions. Below is description of the structure of bovine chymotrypsin. Some additional details in<br /> | ||
| Line 16: | Line 16: | ||
This view shows the <scene name='38/387136/Bovine_chymotrypsin_active_sit/3'>carbonyl group of the inhibitor</scene> in CPK colors. The triflouromethyl group is bound to the carbonyl carbon via the yellow bond. In a peptide substrate, the triflouromethyl group would be replaced by the first amino acid residue of the rest of the peptide chain, and the yellow bond would be the bond that is cleaved. The carbonyl carbon of the inhibitor is 1.52 Å away from the side chain oxygen of serine 195, and this indicates they are covalently bound (bond indicated by dotted line). Thus, this structure is similar to the '''tetrahedral intermediate''' that is formed during the cleavage reaction. The negative charge that develops on the carbonyl oxygen of the substrate is stabilized by hydrogen bonds to the backbone nitrogens of Ser 195 and Gly 193, shown in blue spacefill. The hydrogen atoms involved in these hydrogen bonds are not shown. | This view shows the <scene name='38/387136/Bovine_chymotrypsin_active_sit/3'>carbonyl group of the inhibitor</scene> in CPK colors. The triflouromethyl group is bound to the carbonyl carbon via the yellow bond. In a peptide substrate, the triflouromethyl group would be replaced by the first amino acid residue of the rest of the peptide chain, and the yellow bond would be the bond that is cleaved. The carbonyl carbon of the inhibitor is 1.52 Å away from the side chain oxygen of serine 195, and this indicates they are covalently bound (bond indicated by dotted line). Thus, this structure is similar to the '''tetrahedral intermediate''' that is formed during the cleavage reaction. The negative charge that develops on the carbonyl oxygen of the substrate is stabilized by hydrogen bonds to the backbone nitrogens of Ser 195 and Gly 193, shown in blue spacefill. The hydrogen atoms involved in these hydrogen bonds are not shown. | ||
| - | + | </StructureSection> | |
== 3D Structures of Chymotrypsin == | == 3D Structures of Chymotrypsin == | ||
Revision as of 12:31, 9 June 2015
| |||||||||||
3D Structures of Chymotrypsin
Updated on 09-June-2015
The Chy precursor is the inactive chymotrypsinogen (Chygen) which gets cleaved 3 times by trypsin and chymotrypsin losing a 4 amino acid long peptide to become the active Chy. γ-Chy is a covalent acyl adduct of α-Chy. δ-Chy results when Chygen is cleaved only twice.
Further reading
You can learn more about chymotrypsin structure, function and regulation in this publicly available chapter of the Biochemistry textbook by Berg, Tymoczka and Stryer.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alice Harmon, Alexander Berchansky