Sandbox Reserved 1051
From Proteopedia
(Difference between revisions)
| Line 42: | Line 42: | ||
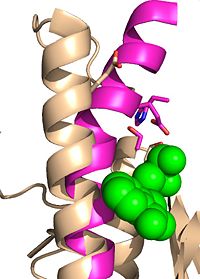
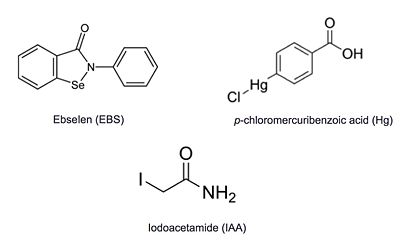
Ag85C can be inhibited by [http://en.wikipedia.org/wiki/Ebselen ebselen], which covalently bounds to the sulfur in C209. Ebselen is a thiol-modifying agent that serves as an electrophile for a C209 nucleophilic attack that results in sulfur-selenium bond formation. Crystallization of ebselen-modified Ag85C provides an explanation for the mechanism of inhibition. The addition of ebselen increases the distance between C209 and L232-T231, which effectively disrupts the interaction that holds the α9 helix in the active conformation. The disruption of this interaction causes the α9 helix to <scene name='69/694220/Inhibited_relaxed_helix/1'>relax</scene>. Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues, which can be seen in '''Figure 5'''. Relaxation of the α9 helix due to ebselen modification moves E228 and H260, which now interacts with S148, instead of S124, in the active site. The displacement of these residues destroys the catalytic triad's charge relay, and as a result, the nucleophilicity of the S124 alcohol is not longer strengthened, which decreases catalytic activity. | Ag85C can be inhibited by [http://en.wikipedia.org/wiki/Ebselen ebselen], which covalently bounds to the sulfur in C209. Ebselen is a thiol-modifying agent that serves as an electrophile for a C209 nucleophilic attack that results in sulfur-selenium bond formation. Crystallization of ebselen-modified Ag85C provides an explanation for the mechanism of inhibition. The addition of ebselen increases the distance between C209 and L232-T231, which effectively disrupts the interaction that holds the α9 helix in the active conformation. The disruption of this interaction causes the α9 helix to <scene name='69/694220/Inhibited_relaxed_helix/1'>relax</scene>. Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues, which can be seen in '''Figure 5'''. Relaxation of the α9 helix due to ebselen modification moves E228 and H260, which now interacts with S148, instead of S124, in the active site. The displacement of these residues destroys the catalytic triad's charge relay, and as a result, the nucleophilicity of the S124 alcohol is not longer strengthened, which decreases catalytic activity. | ||
| - | ====Modification by ''p''-chloromercuribenzoic acid==== | ||
| - | + | ====Modification by ''p''-Chloromercuribenzoic acid==== | |
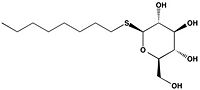
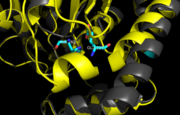
| + | The thiol of C209 can also be covalently modified by [http://en.wikipedia.org/wiki/4-Chloromercuribenzoic_acid p-chloromercuribenzoic acid]. Similar to what is observed in Ag85C-ebselen, the alteration in <scene name='69/694218/4qdo/1'>Ag85C-Hg</scene> relaxes the kinked helix α-9 found in the native structure of the enzyme. The native structure is shown in green and the relaxed, modified structure is shown in blue. This conformational change again disrupts the hydrogen bonding network of the catalytic triad, resulting in a decrease to only 60% of the normal enzymatic function<ref name="Favrot"/>. | ||
| - | [[Image:P-chloromercuribenzoic acid.jpg |100 xp|left|thumb|'''Figure 6b.''' p-chloromercuribenzoic acid]] | ||
| - | |||
| - | |||
| - | The mutant [http://proteopedia.org/wiki/index.php/4qdo Ag85C-Hg] is generated with the addition of [http://en.wikipedia.org/wiki/4-Chloromercuribenzoic_acid p-chloromercuribenzoic acid] (Figure 6), the side chain of the complex is disordered due to a lack of hydrogen bonds between Glu228 and His260. Similar to what is observed in Ag85C-ebselen, the alteration in <scene name='69/694218/4qdo/1'>Ag85C-Hg</scene> relaxes the kinked helix α-9 found in the native structure of the enzyme, thus inhibiting the active site (Figure 5). The ultimate effect is a decrease to only 60% of the normal enzymatic function of Ag85C.<ref name="Favrot"/> | ||
===Mutation of Cys209=== | ===Mutation of Cys209=== | ||
====E228Q==== | ====E228Q==== | ||
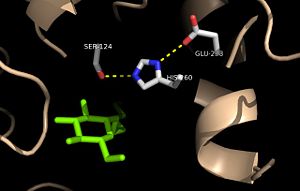
| - | + | Mutation of the glutamate component of the catalytic triad, E228, to glutamine decreased enzymatic activity to only 17% of the wild type. shifted this residue 4 angstroms from its original position in the native structure of Ag85C. Due to the shift of Glu228 is the loss of hydrogen bonds between Ser124 and His260. Instead, <scene name='69/694218/4qdz/2'>His260 bonds with Ser148</scene>, which also results from the shift of Glu228. A weak electron density difference in the His260 position of the native and mutated structures was also noted, suggesting that the residue may take on two alternative conformations in the <scene name='69/694218/Ag85c-e228q/1'>Ag85C-E228Q</scene> mutant. The histidine residue, labeled in pink, takes on two conformations, binding with alternative serine residuesOverall, the enzyme functionality is decreased to only 17% activity.<ref name="Favrot"/> | |
| - | + | ||
| - | + | ||
Unlike other Ag85C mutants and modifications aforementioned, the <scene name='69/694218/Ag85c-e228q/1'>Ag85C-E228Q</scene>does not eliminate any hydrogen bonds in the catalytic triad. Rather, it simply replaces a carboxylate moiety with an amide. Further, the structural change observed in the low-energy conformation of the <scene name='69/694218/Ag85c-e228q/1'>Ag85C-E228Q</scene> mutant provides additional support for the hypothesis that the natively kinked helix α-9 is central to the enzymatic function of Ag85C.<ref name="Favrot"/> | Unlike other Ag85C mutants and modifications aforementioned, the <scene name='69/694218/Ag85c-e228q/1'>Ag85C-E228Q</scene>does not eliminate any hydrogen bonds in the catalytic triad. Rather, it simply replaces a carboxylate moiety with an amide. Further, the structural change observed in the low-energy conformation of the <scene name='69/694218/Ag85c-e228q/1'>Ag85C-E228Q</scene> mutant provides additional support for the hypothesis that the natively kinked helix α-9 is central to the enzymatic function of Ag85C.<ref name="Favrot"/> | ||
Revision as of 20:45, 15 June 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
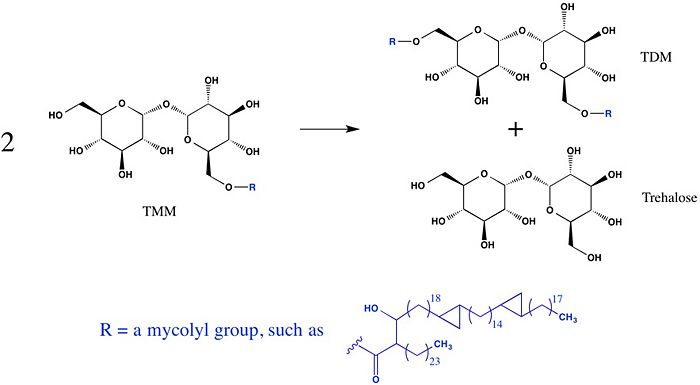
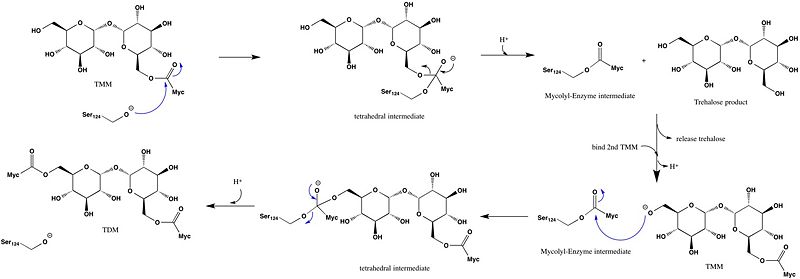
Trehalose-O-mycolyltransferase Ag85C
Introduction
Antigen 85C is one of three homologous protein components of the Ag85 complex in the cell wall of M. tuberculosis. This serine esterase enzyme catalyzes the transfer of mycolyl groups, characteristic components of the cell wall of mycobacteria. Several three dimensional structures of Ag85C have been solved, including the wild type enzyme as well as active site variants due to site-directed mutagenesis and covalent modification.
| |||||||||||
References
- ↑ Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999 Mar;31(5):1573-87. PMID:10200974
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413
- ↑ Ronning DR, Vissa V, Besra GS, Belisle JT, Sacchettini JC. Mycobacterium tuberculosis antigen 85A and 85C structures confirm binding orientation and conserved substrate specificity. J Biol Chem. 2004 Aug 27;279(35):36771-7. Epub 2004 Jun 10. PMID:15192106 doi:http://dx.doi.org/10.1074/jbc.M400811200
- ↑ Favrot L, Lajiness DH, Ronning DR. Inactivation of the Mycobacterium tuberculosis Antigen 85 complex by covalent, allosteric inhibitors. J Biol Chem. 2014 Jul 14. pii: jbc.M114.582445. PMID:25028518 doi:http://dx.doi.org/10.1074/jbc.M114.582445
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedFavrot