Sandbox Reserved 1076
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | =ESX | + | =ESX Secretion-Associated Protein G (EspG)= |
==Introduction== | ==Introduction== | ||
| Line 9: | Line 9: | ||
==Binding Specificity of EspG5 to PE25-PPE41 Proteins in ''Mycobacterium tuberculosis''== | ==Binding Specificity of EspG5 to PE25-PPE41 Proteins in ''Mycobacterium tuberculosis''== | ||
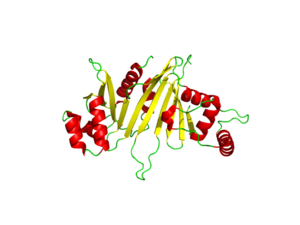
[[Image:EspG3_White.png|300 px|left|thumb|Figure 1: EspG3 protein (from [http://proteopedia.org/wiki/index.php/4w4i 4w4i])]] | [[Image:EspG3_White.png|300 px|left|thumb|Figure 1: EspG3 protein (from [http://proteopedia.org/wiki/index.php/4w4i 4w4i])]] | ||
| - | + | ==Biological Function== | |
| + | Through specific binding factors, an EspG binds to its PE-PPE ligand to be secreted through the ESAT pathway. | ||
| + | ==Clinical Relevance== | ||
| + | Though the ESAT-6 secretion system is poorly understood, it is known that PE-PPE proteins and EspG proteins influence virulence and pathogenicity of the infection. | ||
== General Structure and Function == | == General Structure and Function == | ||
| - | The EspG<sub>3</sub> protein shown in Figure 1 has a mass of 33.7kD <ref>PMID:25275011</ref>. The | + | The EspG<sub>3</sub> protein shown in Figure 1 has a mass of 33.7kD <ref name="Ekiert2014">PMID:25275011</ref>. The protein's beta sheets (yellow) make up the core of the protein. The 2-fold pseudo-symmetry implied by this cartoon view of the secondary structure is not supported by sequence similarity. As a monomeric protein, this binds to its ligand with high specificity. A key beta sheet region on the <scene name='69/694242/Espg3_differences/4'>β-2 and β-3 strands</scene> will vary between EspG subtypes to influence what the random loop on the PE-PPE protein will interact with, since this region includes key residues that interact with the random coil of the [http://proteopedia.org/wiki/index.php/PE/PPE_Protein_Complex PE-PPE] ligand. The β-sheet core is surrounded by 8 alpha helices (red). One key <scene name='69/694242/Espg3_differences/2'>alpha helix</scene> will vary among different EspG proteins to stericly limit binding to PE-PPE ligand. Variations in the long <scene name='69/694242/Espg3_differences/3'>random loop</scene> can also impact binding to the EspG's ligand. |
| - | |||
| - | Through specific binding factors, an EspG binds to its PE-PPE ligand to be secreted through the ESAT pathway. Though the ESAT-6 secretion system is poorly understood, it is known that PE-PPE proteins and EspG proteins influence virulence and pathogenicity of the infection. | ||
==Structural Differences between EspG subtypes== | ==Structural Differences between EspG subtypes== | ||
Revision as of 13:52, 16 June 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
ESX Secretion-Associated Protein G (EspG)
Introduction
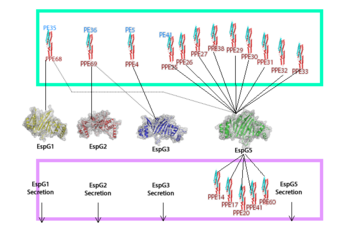
EspG is a key secretion protein involved with the virulence of Mycobacterium tuberculosis. The specificity of EspG binding affinity to its specific PE-PPE ligand has many contributing factors. The four different EspG proteins found in Mycobacterium tuberculosis have different characteristics that influence binding, where EspG5 binds to the most PE-PPE proteins. Not all EspG proteins bind to the same ligand; specific interactions from specific residue interactions, electrostatics, steric hinderance and concavity of the EspG binding pocket influence binding. The EspG PE-PPE complex is to be excreted in the ESAT-6 pathway, this pathway is an attractive target for inducing apoptosis in Mtb, this makes it a good drug target.
| |||||||||||
References
- ↑ Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14758-63. doi:, 10.1073/pnas.1409345111. Epub 2014 Oct 1. PMID:25275011 doi:http://dx.doi.org/10.1073/pnas.1409345111
- ↑ Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 2005 Jul 20;24(14):2491-8. Epub 2005 Jun 23. PMID:15973432
Similar Pages
Student Contributors
- Mark Meredith
- Jonathan Golliher