We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
PLC beta 3 Gq
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
'''Overview of the PLC-β3 and Gq interface:''' | '''Overview of the PLC-β3 and Gq interface:''' | ||
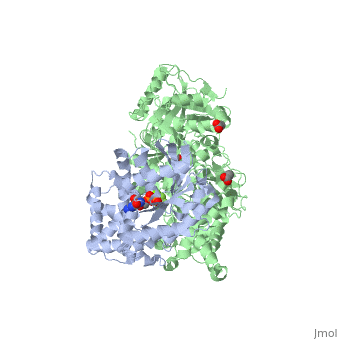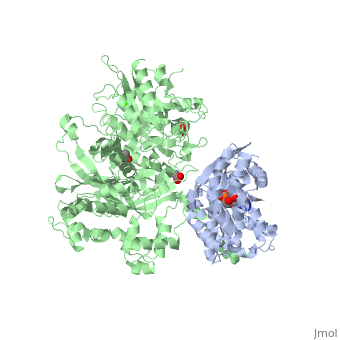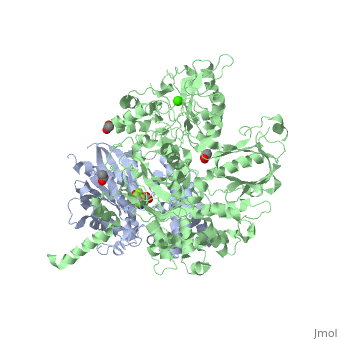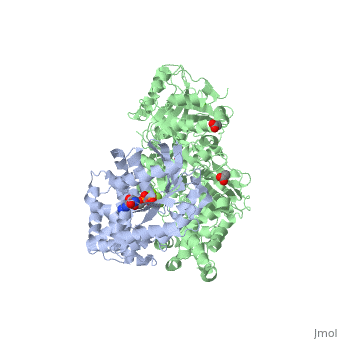
| - | The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called <scene name='70/701452/Fig2/6'>switch regions I-III</scene>.These regions allows the Gαq to be released from the receptor and activate distinct downstream effectors, | + | The Gαq subunit consists two domains, one is the GTPase domain and the other is the alpha helical domain. These domains include three regions called <scene name='70/701452/Fig2/6'>switch regions I-III</scene>.These regions allows the Gαq to be released from the receptor and activate distinct downstream effectors, which carry on the signal to downstream targets. A central effector of Gαq being the PLC-β3 enzyme. The switch regions I and II interact with several domains in PLC-β3. |
<scene name='70/701452/Fig1/9'>The PLC-β3 has several domains</scene> consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel that the X/Y linker connect its two halves and a C2 domain. | <scene name='70/701452/Fig1/9'>The PLC-β3 has several domains</scene> consisting of N-terminal PH domain, a series of four EF hands, a catalytic TIM barrel that the X/Y linker connect its two halves and a C2 domain. | ||
| Line 17: | Line 17: | ||
'''Local motifs in Gq-PLC-β3 interactions:''' | '''Local motifs in Gq-PLC-β3 interactions:''' | ||
| - | <scene name='70/701452/Fig6/5'>The | + | <scene name='70/701452/Fig6/5'>The 703-725 loop</scene> between the end of the TIM barrel and the beginning of the C2 domain comprises a second distinct segment of PLC-β3 that makes extensive contacts with active Gαq, including switches 1 and 2. This interface includes a series of interdigitated pairs of charged residues, specifically in PLC-β3-Gαq Asp709/Arg202, Lys710/Glu191, and Asp721/Lys41; these in turn are supported by additional charged residues Glu703 and Arg707 of PLC- β3. |
<scene name='70/701452/Fig7/1'>An extended loop (residues 254-268)</scene> between EF hands 3 and 4 of PLC-β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | <scene name='70/701452/Fig7/1'>An extended loop (residues 254-268)</scene> between EF hands 3 and 4 of PLC-β3 interacts with the GTP-binding region of Gαq. Asn260 of the EF3/4 loop promotes GTP hydrolysis by interaction with the side chain of Gln209 of Gαq, which rearranges during GTP hydrolysis to stabilize the transition state mimicked by GDP•AlF4–•H20. Asn260 also interacts with Glu212 to stabilize switch 1 for GTP hydrolysis. | ||
| - | Other effectors are known to engage | + | Other effectors are known to engage <scene name='70/701452/Fig3/3'>different regions (pink)</scene> <ref>PMID:20966218</ref> in Gαq within the Gα subunits. There are a large family of regulator of G protein signaling (RGS) proteins that independently accelerates the GTP hydrolysis , these RGS proteins engage the GTPase domain throughout <scene name='70/701452/Fig4/3'>several residues (brown)</scene> on Gαq . PLC-β3 interacts with <scene name='70/701452/Fig5/4'>residues (blue)</scene> on Gαq that overlaps almost completely with portions of Gα subunits needed for engagement of RGS proteins and other effectors. |
'''Critical residues in the interface:''' | '''Critical residues in the interface:''' | ||
Revision as of 17:22, 21 July 2015
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
| |||||||||||
References
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438