We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
FMDV 3C
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
'''By, Erica Martel''' | '''By, Erica Martel''' | ||
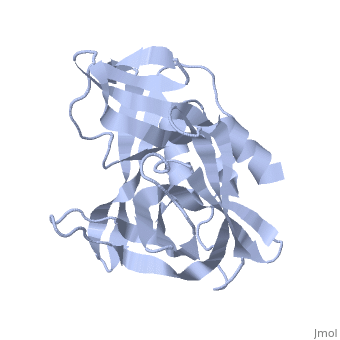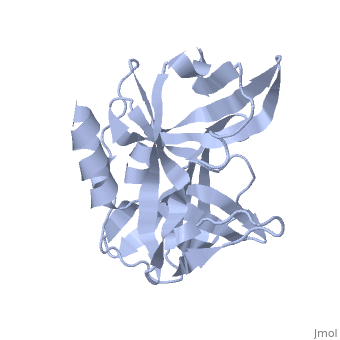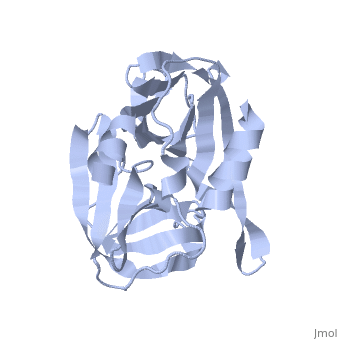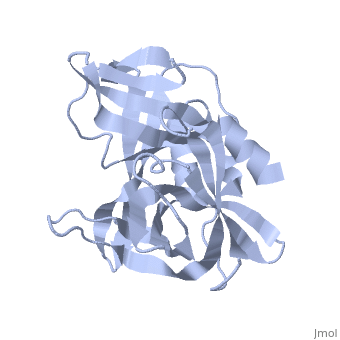
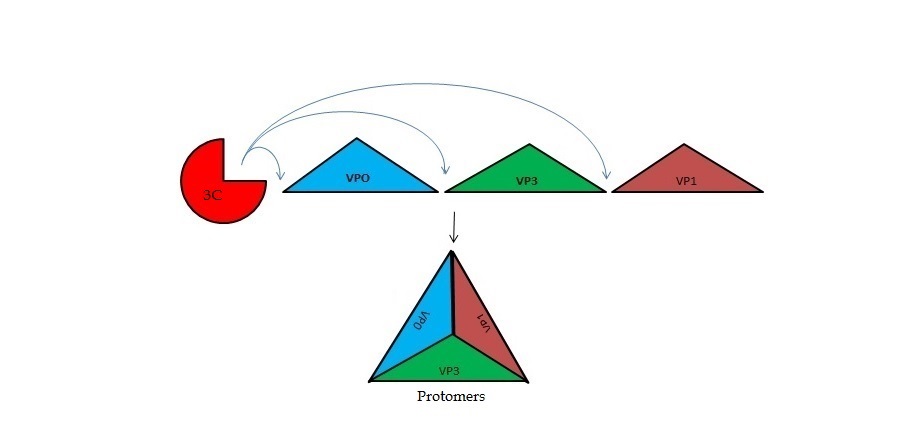
| - | The FMDV viral genome is translated as a single polypeptide precursor that must be cleaved into functional proteins by virally encoded proteases. The FMDV 3C protease is responsible for most cleavages of the viral polyprotein (10 out of the 13 cleavages) and has a highly conserved amino acid sequence across different viral serotypes, making the enzyme an attractive target for inhibitor design and antiviral drugs. The 3C protease cuts itself out of the | + | The FMDV viral genome is translated as a single polypeptide precursor that must be cleaved into functional proteins by virally encoded proteases. The FMDV 3C protease is responsible for most cleavages of the viral polyprotein (10 out of the 13 cleavages) and has a highly conserved amino acid sequence across different viral serotypes, making the enzyme an attractive target for inhibitor design and antiviral drugs. The 3C protease cuts itself out of the polyprotein and is responsible for processing the P1 peptide into three viral peptides, VP0 VP3, and VP1. The VP0 peptide is further processed into VP4 and VP2 by an unknown mechanism. To form the FMDV capsid fully processed VPs assemble into a triangular protomer, Figure below. Five protomers come together to make a pentamer, twelve pentamers conjoin to complete the virus shell. |
Revision as of 15:38, 28 July 2015
FMDV 3C Protease
| |||||||||||
References
references [1]