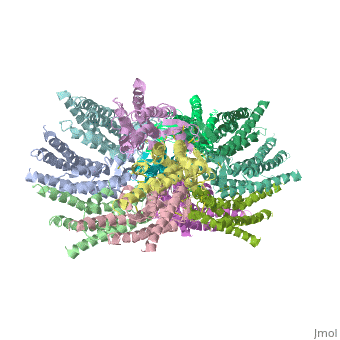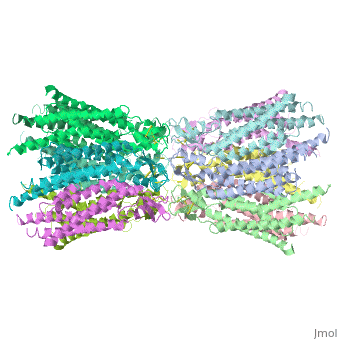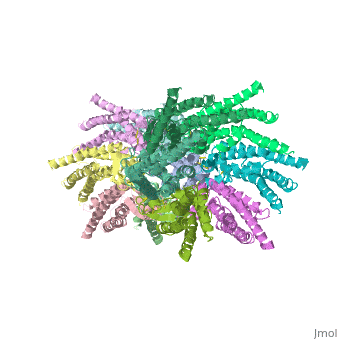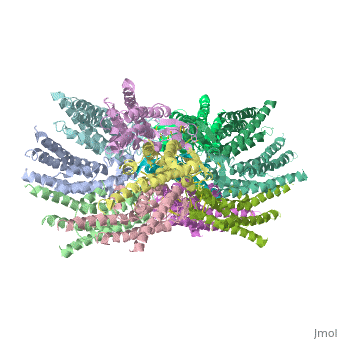
We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Connexin
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
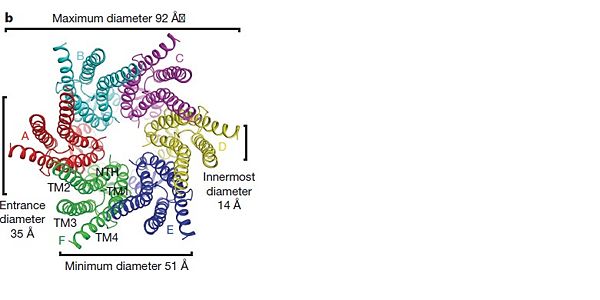
| - | The transmembrane region of the channel is 38Å thick.TM2 extends about 19Å from the membrane surface into the cytoplasm. The extracellular region of the connexon extends 23Å from the membrane surface and interdigitates to the opposite connexon by 6Å, resulting in the intercellular ‘gap’ of 40Å. The extracellular lobes are not protruding so much, as indicated by the structural analyses of split gap junction channels with atomic force microscopy and electron microscopy. The relatively flat lobes could be attributed to the conformational change of the extracellular region induced by the docking of two connexons. The diameter of the connexon is biggest at the cytoplasmic side of the membrane, 92Å , and smallest at the extracellular side, 51Å . | + | The transmembrane region of the channel is 38Å thick. TM2 extends about 19Å from the membrane surface into the cytoplasm. The extracellular region of the connexon extends 23Å from the membrane surface and interdigitates to the opposite connexon by 6Å, resulting in the intercellular ‘gap’ of 40Å. The extracellular lobes are not protruding so much, as indicated by the structural analyses of split gap junction channels with atomic force microscopy and electron microscopy. The relatively flat lobes could be attributed to the conformational change of the extracellular region induced by the docking of two connexons. The diameter of the connexon is biggest at the cytoplasmic side of the membrane, 92Å , and smallest at the extracellular side, 51Å . |
Viewed from the top, the channel looks like a ‘hexagonal nut’ with a pore in the centre .The diameter of the pore is about 40Å at the cytoplasmic side of the channel, narrowing to 14Å near the extracellular membrane surface and then widening to 25Å in the extracellular space.<ref name='Structure'/> | Viewed from the top, the channel looks like a ‘hexagonal nut’ with a pore in the centre .The diameter of the pore is about 40Å at the cytoplasmic side of the channel, narrowing to 14Å near the extracellular membrane surface and then widening to 25Å in the extracellular space.<ref name='Structure'/> | ||
[[Image:distances b.jpg | thumb |600px | center | Top view of the Cx26 gap junction channel]] | [[Image:distances b.jpg | thumb |600px | center | Top view of the Cx26 gap junction channel]] | ||
| Line 30: | Line 30: | ||
==Structure of the cx26 protomer:== | ==Structure of the cx26 protomer:== | ||
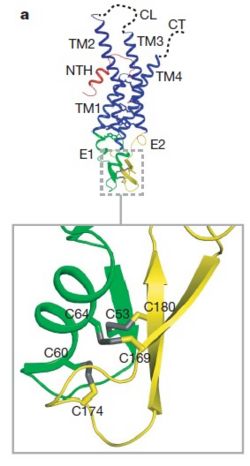
As mentioned before <scene name='70/701426/Connexin_structure/1'>the connexin</scene> [http://en.wikipedia.org/wiki/Promoter_(genetics) protomer] has four transmembrane (TM1–4), two extracellular loops , a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment. Cx26 forms a typical four-helix bundle in which any pair of adjacent helices is antiparallel. The major pore-lining helix TM1 is inclined, so that the pore diameter narrows from the cytoplasmic to the extracellular side of the membrane, and ends in a short [http://en.wikipedia.org/wiki/310_helix 3<sub>10</sub> helix]. | As mentioned before <scene name='70/701426/Connexin_structure/1'>the connexin</scene> [http://en.wikipedia.org/wiki/Promoter_(genetics) protomer] has four transmembrane (TM1–4), two extracellular loops , a cytoplasmic loop, an N-terminal helix (NTH), and a C-terminal segment. Cx26 forms a typical four-helix bundle in which any pair of adjacent helices is antiparallel. The major pore-lining helix TM1 is inclined, so that the pore diameter narrows from the cytoplasmic to the extracellular side of the membrane, and ends in a short [http://en.wikipedia.org/wiki/310_helix 3<sub>10</sub> helix]. | ||
| - | The extracellular loop E1 contains a [http://en.wikipedia.org/wiki/310_helix 3<sub>10</sub> helix] at the beginning and a short α-helix in its C-terminal. E2, together with E1, contains a short antiparallel β-sheet and stretches over E1, forming the outside <scene name='70/701426/Connexon_bachbone/1'>wall</scene> of the connexon. Six conserved cysteine residues, three in each loop, form intramolecular disulphide bonds between E1 and E2 Most of the prominent intra-protomer interactions are in the extracellular part of the transmembrane region, The interactions between the two adjoining connexons of the gap junction channel, which involve both E1 and E2 .[[image:E1.jpg | thumb | center | 250px | Disulfide bonds between two extracellular loops in the Cx26 promoter]] | + | The extracellular loop E1 contains a [http://en.wikipedia.org/wiki/310_helix 3<sub>10</sub> helix] at the beginning and a short α-helix in its C-terminal. E2, together with E1, contains a short antiparallel β-sheet and stretches over E1, forming the outside <scene name='70/701426/Connexon_bachbone/1'>wall</scene> of the connexon. Six conserved cysteine residues, three in each loop, form intramolecular disulphide bonds between E1 and E2 Most of the prominent intra-protomer interactions are in the extracellular part of the transmembrane region, The interactions between the two adjoining connexons of the gap junction channel, which involve both E1 and E2. [[image:E1.jpg | thumb | center | 250px | Disulfide bonds between two extracellular loops in the Cx26 promoter]] |
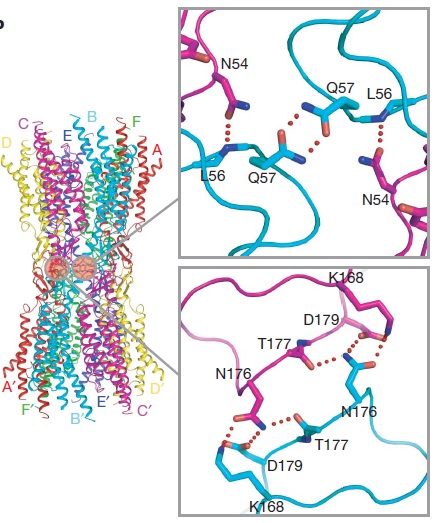
The N-terminal half of E2 seems rather flexible and its amino-acid sequence varies greatly among connexins . The C-terminal half of E2 begins with a 310 turn is followed by a conserved Pro-Cys-Pro motif that reverses its direction back to TM4. Most of the prominent intra-protomer interactions are in the extracellular part of the transmembrane region . Arg 32 (TM1) interactswithGln 80 (TM2),Glu 147 (TM3), and Ser 199 (TM4). Two hydrophobic cores around Trp 44 (E1) and Trp 77 (TM2) stabilize the protomer structure. Ala 39 (TM1), Ala 40 (TM1), Val 43 (E1) and Ile 74 (TM2) contribute to the first hydrophobic core around Trp 44, and Phe 154 (TM3) and Met 195 (TM4) form the second core with Trp 77 . In the intracellular part of the transmembrane region, Arg 143 (TM3) forms hydrogen bonds with Asn 206 (TM3) and Ser 139 (TM3) .<ref name='Structure'/> | The N-terminal half of E2 seems rather flexible and its amino-acid sequence varies greatly among connexins . The C-terminal half of E2 begins with a 310 turn is followed by a conserved Pro-Cys-Pro motif that reverses its direction back to TM4. Most of the prominent intra-protomer interactions are in the extracellular part of the transmembrane region . Arg 32 (TM1) interactswithGln 80 (TM2),Glu 147 (TM3), and Ser 199 (TM4). Two hydrophobic cores around Trp 44 (E1) and Trp 77 (TM2) stabilize the protomer structure. Ala 39 (TM1), Ala 40 (TM1), Val 43 (E1) and Ile 74 (TM2) contribute to the first hydrophobic core around Trp 44, and Phe 154 (TM3) and Met 195 (TM4) form the second core with Trp 77 . In the intracellular part of the transmembrane region, Arg 143 (TM3) forms hydrogen bonds with Asn 206 (TM3) and Ser 139 (TM3) .<ref name='Structure'/> | ||
[[image:bonds.jpg | thumb | center | 500px | Intracellular interactions]] | [[image:bonds.jpg | thumb | center | 500px | Intracellular interactions]] | ||
| Line 38: | Line 38: | ||
In general, single site mutations are spread fairly evenly across the whole protein with TM2 having the highest mutation density (number of amino acids with NHLS mutations divided by the total number of amino acids in the domain) at 67% to M1 and E1, having the lowest density of mutations with their respective domains at 33%. According to this criterion, TM4 has a mutation density of 40%. Of the four transmembrane helices, M1, M2 and M3 have attracted the most attention, because of the controversies involved in models with different helix assignments, based on lower resolution cryo-electron crystallographic structures and scanning cysteine accessibility mutagenesis. Far less is known about TM4 and how side chains interact with the other helices and with the lipid bilayer. <ref name='mutant int'/> | In general, single site mutations are spread fairly evenly across the whole protein with TM2 having the highest mutation density (number of amino acids with NHLS mutations divided by the total number of amino acids in the domain) at 67% to M1 and E1, having the lowest density of mutations with their respective domains at 33%. According to this criterion, TM4 has a mutation density of 40%. Of the four transmembrane helices, M1, M2 and M3 have attracted the most attention, because of the controversies involved in models with different helix assignments, based on lower resolution cryo-electron crystallographic structures and scanning cysteine accessibility mutagenesis. Far less is known about TM4 and how side chains interact with the other helices and with the lipid bilayer. <ref name='mutant int'/> | ||
| - | Two structural crystallographic studies have been commenced on Cx26, the first one describing the WT protein in a resolution of 3.5 A⁰, and the second one deals with two types of mutations in the N terminus of the protein. | + | Two structural crystallographic studies have been commenced on Cx26, the first one describing the WT protein in a resolution of 3.5 A⁰ by Tsuhikara, T. (2009,nature. PMID 19622859), and the second one deals with two types of mutations in the N terminus of the protein by Fujiyoshi, Y. (2011,J.MOL.BIOL. PMID 21094651). |
Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the <scene name='70/701426/N_terminal/1'>N terminus</scene> (blue coloured) as a key component in gating, and possibly assembly. <ref name='pdb'>pmid 21094651</ref> | Gap junction channels are unique in that they possess multiple mechanisms for channel closure, several of which involve the <scene name='70/701426/N_terminal/1'>N terminus</scene> (blue coloured) as a key component in gating, and possibly assembly. <ref name='pdb'>pmid 21094651</ref> | ||
The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | ||
Revision as of 08:06, 2 August 2015
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Safaa Salah Hussiesy, Michal Harel, Doaa Naffaa, Jaime Prilusky