Regulator of G protein signaling
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
The α subunits function as guanine nucleotide on-off switches, mechanistically similar to other G-proteins that are enzymatic GTPases. | The α subunits function as guanine nucleotide on-off switches, mechanistically similar to other G-proteins that are enzymatic GTPases. | ||
G-proteins interact with diverse protein partners, such as G-protein coupled receptors (GPCRs), downstream effectors, and other proteins. | G-proteins interact with diverse protein partners, such as G-protein coupled receptors (GPCRs), downstream effectors, and other proteins. | ||
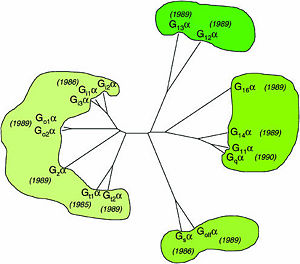
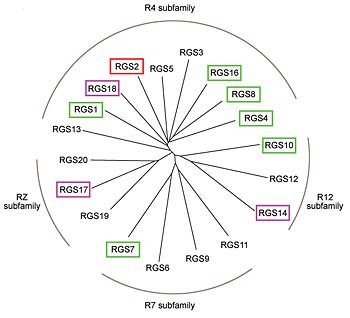
| - | One important G-protein interaction is with members of the RGS protein family. This interaction occurs when the G-protein alpha subunit is activated, and depends on the Gα class, which in turn depends on their sequence that classifies them into several sub-types. Based on the Phylogenetic tree of mammalian G-protein, G-protein α-subunits classified to 4 groups based on | + | One important G-protein interaction is with members of the RGS protein family. This interaction occurs when the G-protein alpha subunit is activated, and depends on the Gα class, which in turn depends on their sequence that classifies them into several sub-types. Based on the Phylogenetic tree of mammalian G-protein, G-protein α-subunits are classified to 4 groups based on their sequence identity. The first two groups, Gα<sub>i</sub> and Gα<sub>q</sub> families have selectivity towards The RGS members of R4-subfamily and R12-subfamily. Another group, Gα<sub>z</sub> subunits were suggested to have selectivity for the RGS members of RZ-subfamily. The last subfamilies Gα<sub>s</sub> and Gα<sub>12/13</sub> might interact with diverse proteins subfamilies that include the ~120-residue RGS homology domain but can't interact with canonical RGS members. |
<imagemap> | <imagemap> | ||
Image:Ga_family_figure1-heterotrimeric_G-protein-short_history_06.jpg|300px|Homology of mammalian G-protein α-subunits. | Image:Ga_family_figure1-heterotrimeric_G-protein-short_history_06.jpg|300px|Homology of mammalian G-protein α-subunits. | ||
poly 131 45 213 41 210 110 127 109 [[2zv4]] | poly 131 45 213 41 210 110 127 109 [[2zv4]] | ||
</imagemap> | </imagemap> | ||
| - | Phylogenetic tree of mammalian G-protein α-subunits classified to 4 groups based on | + | Phylogenetic tree of mammalian G-protein α-subunits classified to 4 groups based on their sequence identity.<ref name="Milligan2006">PMID: 16402120</ref> |
== RGS proteins == | == RGS proteins == | ||
Revision as of 08:51, 6 August 2015
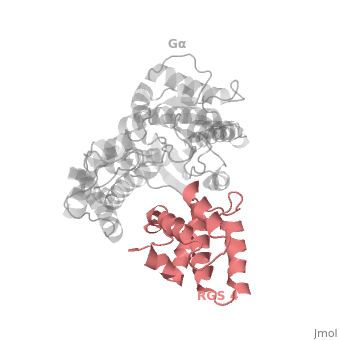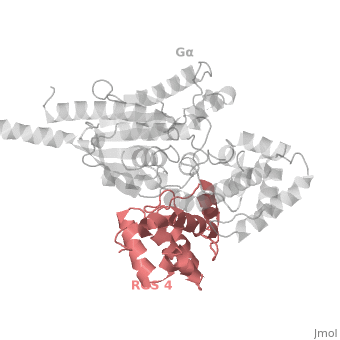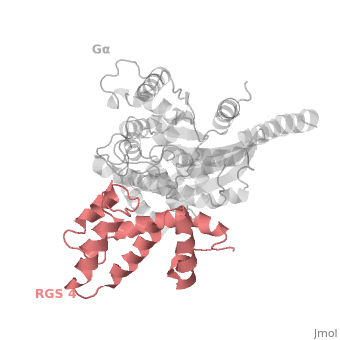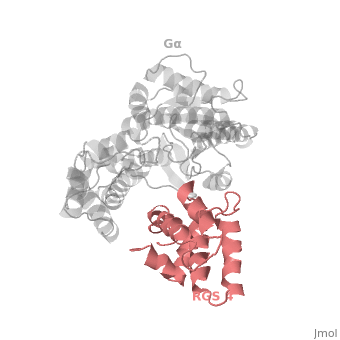
Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gαi as a model structure.
| |||||||||||
References
- ↑ Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147 Suppl 1:S46-55. PMID:16402120 doi:http://dx.doi.org/10.1038/sj.bjp.0706405
- ↑ 2.0 2.1 2.2 Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
- ↑ 3.0 3.1 Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997 Apr 18;89(2):251-61. PMID:9108480
Proteopedia Page Contributors and Editors (what is this?)
Ali Asli, Denise Salem, Michal Harel, Joel L. Sussman, Jaime Prilusky