We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
SCF-KIT
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Function == | == Function == | ||
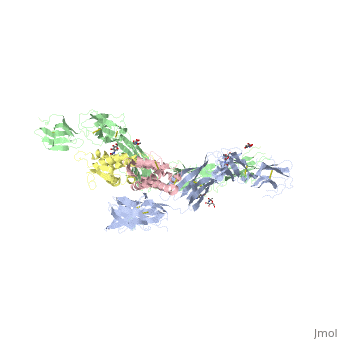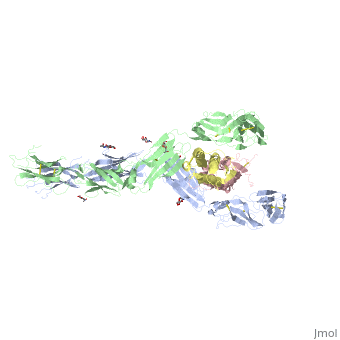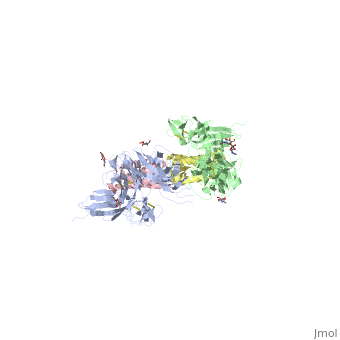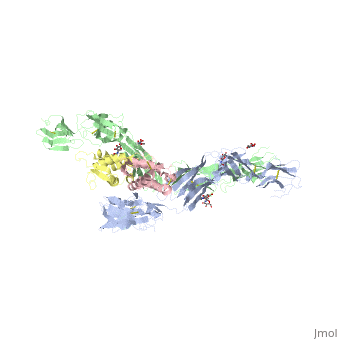
| - | Dimerization of KIT is also mediated by homotypic interactions between the two membrane-proximal Ig-like domains of KIT, namely by D4-D4 and D5-D5 interactions. This results in a significant change in the configurations of D4 and D5 relative to the rest of the molecule. These configurations bring the C termini of the two neighboring ectodomains within 15 | + | Dimerization of KIT is also mediated by homotypic interactions between the two membrane-proximal Ig-like domains of KIT, namely by D4-D4 and D5-D5 interactions. This results in a significant change in the configurations of D4 and D5 relative to the rest of the molecule. These configurations bring the C termini of the two neighboring ectodomains within 15 Å of each other, close to the place where they connect to the transmembrane domain. |
SCF-KIT interface can be divided to three: SiteI, Site II and Site III. | SCF-KIT interface can be divided to three: SiteI, Site II and Site III. | ||
The dimerization of KIT is made possible by <scene name='70/702908/Scf_bound_to_kit/1'>bivalent SCF binding,</scene> whose sole function is to bind SCF and to bring together two KIT molecules. This dimerization is followed by a large change in D4 and D5 orientations. It was proposed that the flexible joints at the D3-D4 and D4-D5 interfaces enable lateral interactions that result in a large conformational change upon receptor dimerization. In the process of dimerization, there is a selection between particular conformations in a transition from a flexibly jointed monomer to a rigid dimer. | The dimerization of KIT is made possible by <scene name='70/702908/Scf_bound_to_kit/1'>bivalent SCF binding,</scene> whose sole function is to bind SCF and to bring together two KIT molecules. This dimerization is followed by a large change in D4 and D5 orientations. It was proposed that the flexible joints at the D3-D4 and D4-D5 interfaces enable lateral interactions that result in a large conformational change upon receptor dimerization. In the process of dimerization, there is a selection between particular conformations in a transition from a flexibly jointed monomer to a rigid dimer. | ||
Revision as of 19:06, 17 August 2015
| |||||||||||