Ivan Koutsopatriy estrogen receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
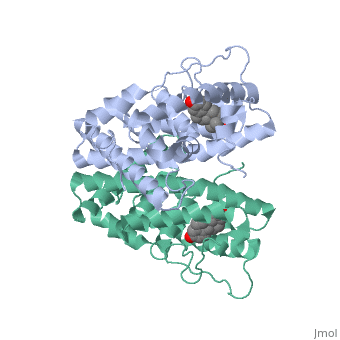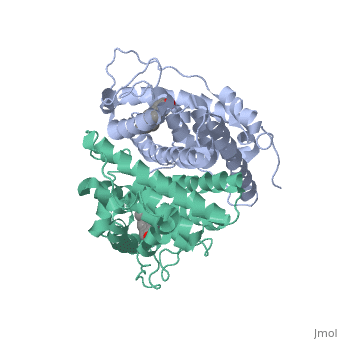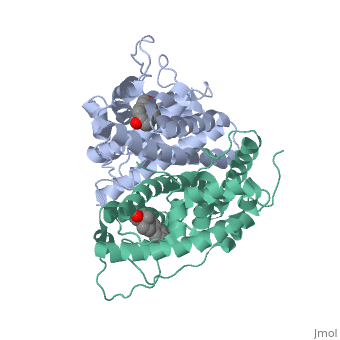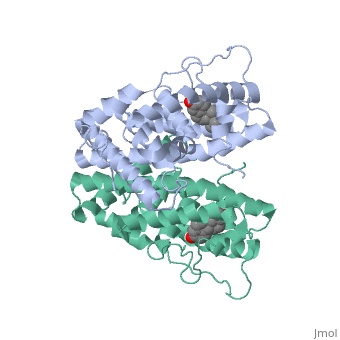
<StructureSection load='1qku' size='340' side='right' caption='Estrogen Receptor with estradiol bound (estradiol is not seen in the picture, because it is bound under the loop)=''> | <StructureSection load='1qku' size='340' side='right' caption='Estrogen Receptor with estradiol bound (estradiol is not seen in the picture, because it is bound under the loop)=''> | ||
| - | There are two flavors of estrogen receptors. ER - Intracellular receptor and GPER | + | There are two flavors of estrogen receptors. ER - Intracellular receptor and GPER - G protein coupled receptors. ERs are transcription factors while GPER are not transcription factors. ERs are the focus of this page. JSmol in Proteopedia was used to help create images <ref>DOI 10.1002/ijch.201300024</ref> and the article describing Jmol, Jmol <ref>PMID:21638687</ref> to the rescue was utilized in the production of this paper. |
| Line 9: | Line 9: | ||
== Structural highlights == | == Structural highlights == | ||
| - | ER is a modular protein composed of a ligand binding domain, a DNA binding domain and | + | ER is a modular protein composed of a ligand binding domain, a DNA binding domain and a transactivation domain. ER is a DNA-binding transcription factor. <scene name='71/714947/Er_bound_to_dna_dnadomain/1'> ER bound to DNA with one DNA binding helix and the transactivation domain highlighted yellow </scene> The DNA binding domain can be clearly observed in this scene with the highlighted yellow helix in close proximety to the DNA. The transactivation domain is attached at the end of the DNA binding domain, also colored in yellow. The transactivation domain activates RNA polymerase when the receptor binds to DNA. The ligand binding domain may be observed here with the following scene. <scene name='71/714947/Agonist_ferutinine_bound_er/2'> Agonist_ferutinine_bound_er</scene> Unbound ER normally exists loosly around the nucleus; this is subject to change depending on a multitude of factors including cell type, progress through cell cycle and reception of cellular signals. When estrogen enters the cell and binds ER, ER trans-locates and undergoes a conformation shift.<ref> Beato, M., Chavez, S., and Truss, M. (1996). Transcriptional regulation by steroid hormones. Steroids 61: 240–251. </ref> Ligand bound estrogen receptor associates more tightly with the nucleus. |
| + | |||
| - | <scene name='71/714947/Agonist_ferutinine_bound_er/2'>Agonist_ferutinine_bound_er</scene> | ||
Ferutinine also causes ER to form a tight loop allowing stimulation of normal growth. | Ferutinine also causes ER to form a tight loop allowing stimulation of normal growth. | ||
| Line 25: | Line 25: | ||
==DNA Protein Interaction and ER Regulation== | ==DNA Protein Interaction and ER Regulation== | ||
| - | |||
Revision as of 02:57, 27 October 2015
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Beato, M., Chavez, S., and Truss, M. (1996). Transcriptional regulation by steroid hormones. Steroids 61: 240–251.
- ↑ Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG (25 May 2001)."Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity.". J Biol Chem. 276 (21): 18375–83.
- ↑ Htun H, Holth LT, Walker D, Davie JR, Hager GL (1 February 1999). "Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor". Mol Biol Cell 10 (2): 471–86.