Timothy Locksmith sandbox Heat Sock Factor
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Regulation == | == Regulation == | ||
| - | HSF is inhibited by heat shock proteins under normal conditions (regarding temperature). The main regulator of HSF is the temperature of the surrounding environment. When the temperature is high, Hsp’s then associate elsewhere the in the cell with damages structures, and are therefore less likely to bind to HSF. This allows HSP to move to the inside of the nucleus, and interact with it to produce more Hsp’s. Once enough Hsp’s are created, the damage has been taken care of and the temperature has returned to normal, they can once again bind to HSF, and inhibit it from interacting with the DNA | + | HSF is inhibited by heat shock proteins under normal conditions (regarding temperature). The main regulator of HSF is the temperature of the surrounding environment. When the temperature is high, Hsp’s then associate elsewhere the in the cell with damages structures, and are therefore less likely to bind to HSF. This allows HSP to move to the inside of the nucleus, and interact with it to produce more Hsp’s. Once enough Hsp’s are created, the damage has been taken care of and the temperature has returned to normal, they can once again bind to HSF, and inhibit it from interacting with the DNA. |
== Sturcture/DNA Interaction == | == Sturcture/DNA Interaction == | ||
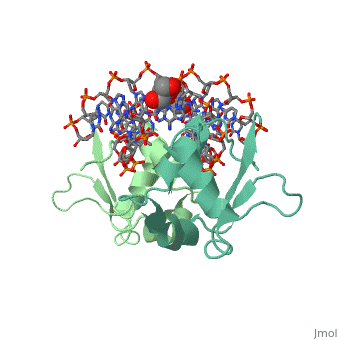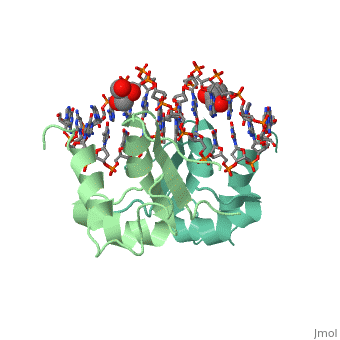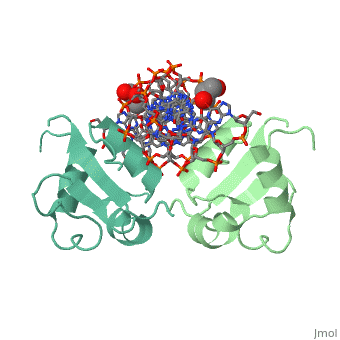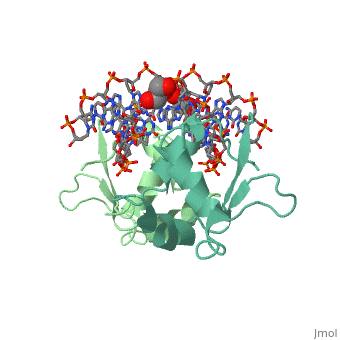
| - | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, trimerize ( <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>HSF_Dimer</scene> could not find representation of trimer), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome. Specifically an Arginine, and Methionine from each of the monomer bind to three oppositely oriented "AGAAN" sections of the genome.2 These residues interact with the nucleotide bases, while nearby residues | + | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, trimerize ( <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>HSF_Dimer</scene> could not find representation of trimer), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome. Specifically an Arginine, and Methionine from each of the monomer bind to three oppositely oriented "AGAAN" sections of the genome.2 These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place <scene name='71/714949/Arg_met_interact/1'>(Arg/Met location)</scene>. |
Revision as of 01:00, 28 October 2015
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269