Timothy Locksmith sandbox Heat Sock Factor
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Sturcture/DNA Interaction == | == Sturcture/DNA Interaction == | ||
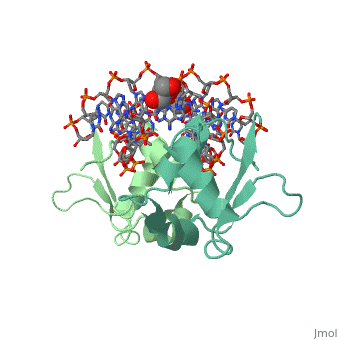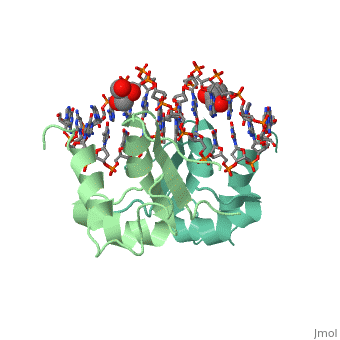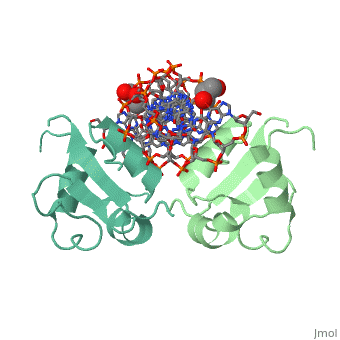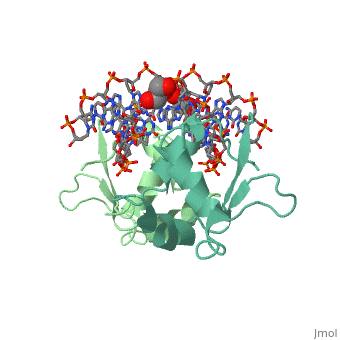
| - | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isloate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/ | + | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isloate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/2'>Arginine, and Methionine</scene> from each of the monomer bind to <scene name='71/714949/Alternating_gaa/2'>three oppositely oriented</scene> "<scene name='71/714949/Gaa_met_arg_interact/2'>nGAAn</scene>" sections of the genome.<ref>PMID:10331875</ref> These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place. The structure is classified as a "winged" helix turn helix |
</structuresection> | </structuresection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 19:04, 28 October 2015
| |||||||||||
References
- ↑ Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS One. 2014 May 6;9(5):e96330. doi: 10.1371/journal.pone.0096330. eCollection , 2014. PMID:24800749 doi:http://dx.doi.org/10.1371/journal.pone.0096330
- ↑ Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230-4. PMID:8421783
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269