Timothy Locksmith sandbox Heat Sock Factor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='3hts' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='3hts' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | |||
== Introduction == | == Introduction == | ||
Heat Shock Factor (HSF) is primarily used for the transcription of Heat shock proteins, which are used for the survival of a cell under high temperature stress situations.<ref>PMID: 24800749</ref> Its structure is highly conserved through species from flies to humans, and even all the way to yeast, which suggests that its existence is very important for the survival of organisms. Studies delving into the uses of HSF have proven promising in Cancer research fields. In some instances when cancer cells had their HSF inhibited underwent cell death instead of proliferation, thus ceasing the spread of the cancer and reducing its size. It is hypothesized that HSF is an integral part of cancer cell survival. | Heat Shock Factor (HSF) is primarily used for the transcription of Heat shock proteins, which are used for the survival of a cell under high temperature stress situations.<ref>PMID: 24800749</ref> Its structure is highly conserved through species from flies to humans, and even all the way to yeast, which suggests that its existence is very important for the survival of organisms. Studies delving into the uses of HSF have proven promising in Cancer research fields. In some instances when cancer cells had their HSF inhibited underwent cell death instead of proliferation, thus ceasing the spread of the cancer and reducing its size. It is hypothesized that HSF is an integral part of cancer cell survival. | ||
| Line 8: | Line 7: | ||
== Sturcture/DNA Interaction == | == Sturcture/DNA Interaction == | ||
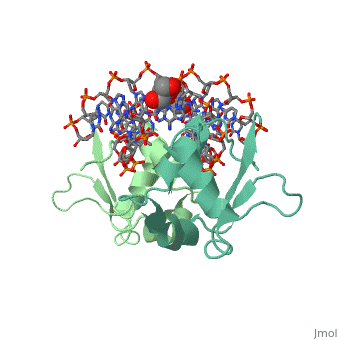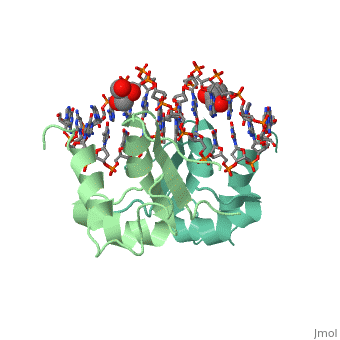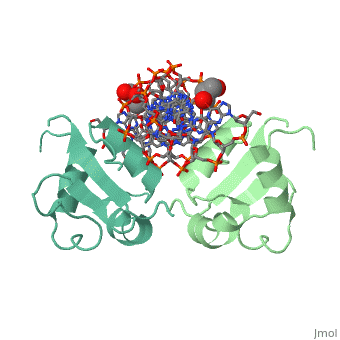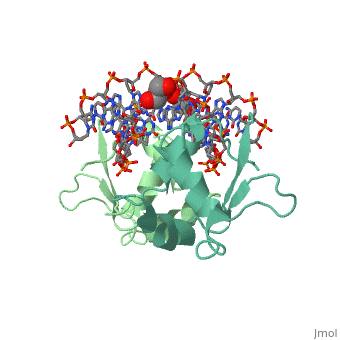
| - | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isloate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/2'>Arginine, and Methionine</scene> from each of the monomer bind to <scene name='71/714949/Alternating_gaa/2'>three oppositely oriented</scene> "<scene name='71/714949/Gaa_met_arg_interact/2'>nGAAn</scene>" sections of the genome.<ref>PMID:10331875</ref> These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place. The structure is classified as a <scene name='71/714949/Dimer_hsf_with_dna_interaction/5'>"winged" helix turn helix</scene> protein. | + | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isloate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/2'>Arginine, and Methionine</scene> from each of the monomer bind to <scene name='71/714949/Alternating_gaa/2'>three oppositely oriented</scene> "<scene name='71/714949/Gaa_met_arg_interact/2'>nGAAn</scene>" sections of the genome.<ref>PMID:10331875</ref> These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place. The structure is classified as a <scene name='71/714949/Dimer_hsf_with_dna_interaction/5'>"winged" helix turn helix</scene> protein.<ref>PMID:10331875</ref> |
</structuresection> | </structuresection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 02:44, 3 November 2015
| |||||||||||
References
- ↑ Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS One. 2014 May 6;9(5):e96330. doi: 10.1371/journal.pone.0096330. eCollection , 2014. PMID:24800749 doi:http://dx.doi.org/10.1371/journal.pone.0096330
- ↑ Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230-4. PMID:8421783
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269