We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Timothy Locksmith sandbox Heat Sock Factor
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Sturcture/DNA Interaction == | == Sturcture/DNA Interaction == | ||
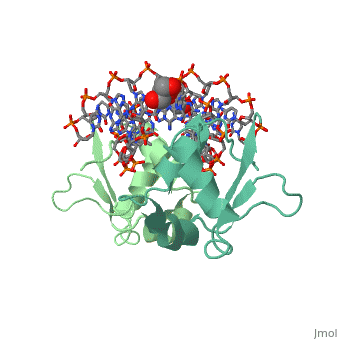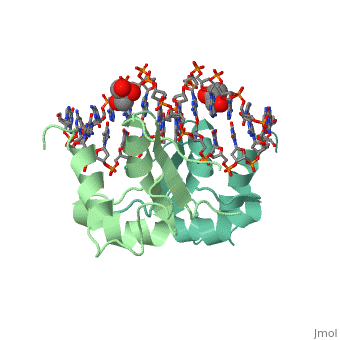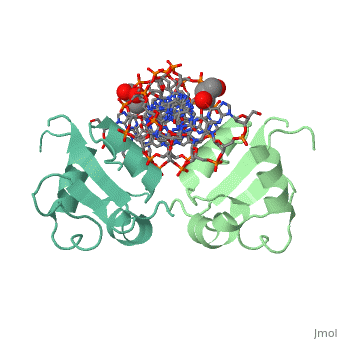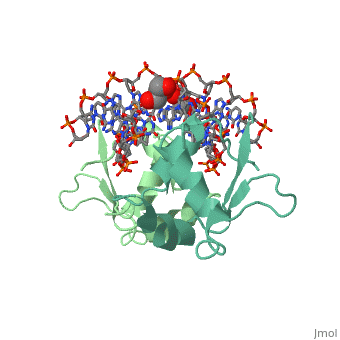
| - | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isolate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/2'>Arginine, and Methionine</scene> from each of the monomer bind to <scene name='71/714949/Alternating_gaa/2'>three oppositely oriented</scene> "<scene name='71/714949/Gaa_met_arg_interact/2'>nGAAn</scene>" sections of the genome.<ref>PMID:10331875</ref> These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place. The structure is classified as a <scene name='71/714949/Dimer_hsf_with_dna_interaction/5'>"winged" helix turn helix</scene> protein. These proteins usually consist of three alpha helices and three beta sheets which are arranged so that one helix interacts with the major groove of DNA while the "wings" interact with the minor groove for further DNA interaction or the backbone for specificity in binding. <ref>PMID:10679470</ref> However this was found to not be the case with HSF, instead the "wings" are used to form the dimer with a second monomer of HSF. | + | HSF remains as a monomer when bound with Hsp under non-stressed conditions. Under stressed conditions (increased temperature) three individual monomers of HSF move into the cell’s nucleus, <scene name='71/714949/Dimer_hsf_with_dna_interaction/3'>trimerizes</scene> (could only isolate a dimer structure), and then bind to the large Groove of the DNA to Heat shock elements throughout the genome.<ref>PMID: 8421783</ref> Specifically an <scene name='71/714949/Arg_met_interact/2'>Arginine, and Methionine</scene> from each of the monomer bind to <scene name='71/714949/Alternating_gaa/2'>three oppositely oriented</scene> "<scene name='71/714949/Gaa_met_arg_interact/2'>nGAAn</scene>" sections of the genome.<ref>PMID:10331875</ref> These residues interact with the nucleotide bases, while nearby residues interact with the back bone to stabilize the transcription factor in place. The structure is classified as a <scene name='71/714949/Dimer_hsf_with_dna_interaction/5'>"winged" helix turn helix</scene> protein. These proteins usually consist of three alpha helices and three beta sheets which are arranged so that one helix interacts with the major groove of DNA while the "wings" interact with the minor groove for further DNA interaction or the backbone for specificity in binding. <ref>PMID:10679470</ref> However this was found to not be the case with HSF, instead the "wings" are used to form the dimer with a second monomer of HSF.[3] |
</structuresection> | </structuresection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 03:02, 3 November 2015
| |||||||||||
References
- ↑ Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS One. 2014 May 6;9(5):e96330. doi: 10.1371/journal.pone.0096330. eCollection , 2014. PMID:24800749 doi:http://dx.doi.org/10.1371/journal.pone.0096330
- ↑ Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230-4. PMID:8421783
- ↑ Littlefield O, Nelson HC. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875 doi:10.1038/8269
- ↑ Gajiwala KS, Burley SK. Winged helix proteins. Curr Opin Struct Biol. 2000 Feb;10(1):110-6. PMID:10679470