Sandbox 77
From Proteopedia
| Line 1: | Line 1: | ||
==Serotonin Receptors== | ==Serotonin Receptors== | ||
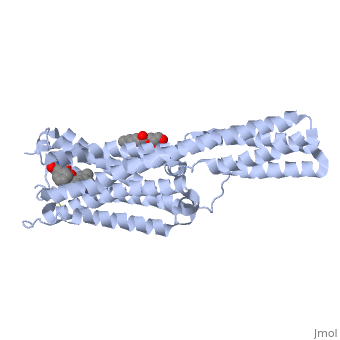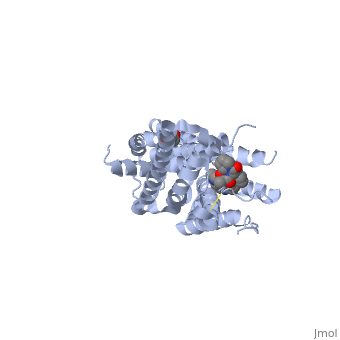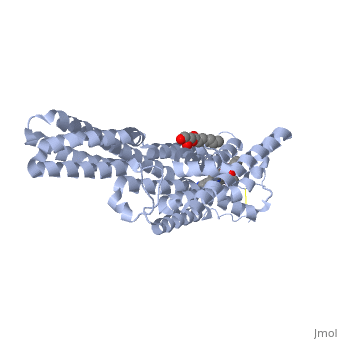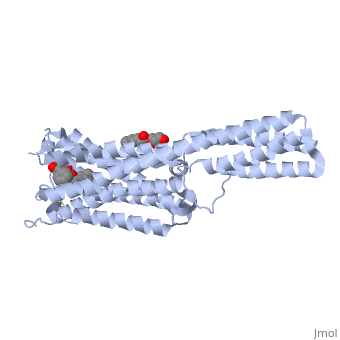
| - | <Structure load='4iar' size=' | + | <Structure load='4iar' size='340' frame='true' align='right' caption='5-HT2B Receptor' scene='' /> |
| - | <Structure load='4ib4' size='340' align='right' caption='5-HT2B Receptor' scene='' /> | + | <Structure load='4ib4' size='340' frame='true' align='right' caption='5-HT2B Receptor' scene='' /> |
<Structure load='4pir' size='340' frame='true' align='right' caption='5-HT3 Receptor' scene='' /> | <Structure load='4pir' size='340' frame='true' align='right' caption='5-HT3 Receptor' scene='' /> | ||
Revision as of 03:26, 16 November 2015
Contents |
Serotonin Receptors
|
|
|
General Function
5-HT, serotonin, receptors are found on the membrane of neurons in the central nervous system and peripheral nervous system. These receptors allow for the body to respond to serotonin and regulate many biological pathways. 5-HT receptors are classified into 7 different subfamilies (5-HT1, 5-HT2, 5-HT3, etc.) by signaling mechanisms and homology of structure. All 5-HT receptors are known to have G-protein linked pathways except for the 5-HT3 receptor which acts as an ion channel. (Wang, et al. 2013).
Structural highlights/Specific Function
5-HT1B: This receptor couples to G-protein alpha subunits Gi and Go. In the central nervous system, this receptor is an inhibitory presynaptic receptor that can alter the release of serotonin, as well as other neurotransmitters, from the presynaptic neuron. The structure of this receptor includes 7 transmembrane alpha-helices in its center. The N-terminal tail of 5-HT1B is close to the ligand binding pocket suggesting some interaction with its ligand. The orthosteric, unmodulated, binding pocket is characterized as a cavity formed from residues of the 3rd, 5th, 6th, and 7th alpha helices and the 2nd extracellular loop. (Wang, et al. 2013)
5-HT2B: This receptor is important in utilizing serotonin signals to encourage proper development and continuing function of the cardiovascular system. Overexpression of 5-HT2B has been linked to congestive heart failure. (Janssen, et al. 2015) 5-HT2B utilizes the alpha Gq protein pathway which triggers intracellular cGMP production through activation of nictric-oxidase synthase (NOS). (Nebigil, et al. 2001) This receptor is also known for being the target of the drug LSD, which has similar structure to serotonin. (Berumen, et al. 2012) The structure of this receptor is much like that of 5-HT1B. It has the characteristic 7 alpha helices and the N-terminus sticks out into extracellular space. (Wang, et al. 2013)
5-HT3: This receptor is a pentameric cation-selective ion channel and plays a role in neuronal excitation to release neurotransmitters from the postsynaptic neuron. 5-HT3 is usually comprised of subunits A or A and B which can result in a homopentameric receptor or a heteropentameric receptor respectively. 5-HT3 is a transmembrane channel that is stimulated to open state by the interaction of the receptor with serotonin in the extracellular space. The binding site is comprised of six loops from two adjacent subunits. The transmembrane region is comprised of multiple alpha helical structures and mediates ion flow and ion specificity. Sodium and Potassium ions are allowed to pass through the 5-HT3 receptor pore. This receptor is involved in the transfer of information to the gastrointestinal tract which makes it a target of drugs which repress vomiting and irritable bowel syndrome. This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
References
Wang, Chong, Yi Jiang, Jinming Ma, Huixian Wu, Daniel Wacker, Vsevolod Katritch, Gye Won Han, Wei Liu, Xi-Ping Huang, Eyal Vardy, John D. McCorvy, Xiang Gao, Edward X. Zhou, Karsten Melcher, Chenghai Zhang, Fang Bai, Huaiyu Yang, Linlin Yang, Hualiang Jiang, Bryan L. Roth, Vadim Cherezov, Raymond C. Stevens, and H. Eric Xu. "Structural Basis for Molecular Recognition at Serotonin Receptors." Science (New York, N.Y.). U.S. National Library of Medicine, 21 Mar. 2013. Web. 15 Nov. 2015. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3644373/
Janssen, Wiebke, Yves Schymura, Tatyana Novoyatleva, Baktybek Kojonazarov, Mario Boehm, Astrid Wietelmann, Himal Luitel, Kirsten Murmann, Damian Richard Krompiec, Aleksandra Tretyn, Soni Savai Pullamsetti, Norbert Weissmann, Werner Seeger, Hossein Ardeschir Ghofrani, and Ralph Theo Schermuly. "5-HT2B Receptor Antagonists Inhibit Fibrosis and Protect from RV Heart Failure." BioMed Research International. Hindawi Publishing Corporation, 1 Feb. 2015. Web. 15 Nov. 2015. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4312574/
Berumen, Laura Cristina, Angelina Rodríguez, Ricardo Miledi, and Guadalupe García-Alcocer. "Serotonin Receptors in Hippocampus." The Scientific World Journal. The Scientific World Journal, 2 May 2012. Web. 15 Nov. 2015. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3353568/
Nebigil, Etienne, Schaerlinger, Hickel, Launay, and Maroteaux. "Developmentally Regulated Serotonin 5-HT2B Receptors." Developmentally Regulated Serotonin 5-HT2B Receptors. Science Direct, 22 May 2001. Web. 15 Nov. 2015. http://www.sciencedirect.com/science/article/pii/S0736574801000223
Thompson, A. J., and S. C. R. Lummis. "5-HT3 Receptors." Current Pharmaceutical Design. U.S. National Library of Medicine, 2 Apr. 2009. Web. 15 Nov. 2015. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2664614/