Sanbox glut3
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Structure== | ==Structure== | ||
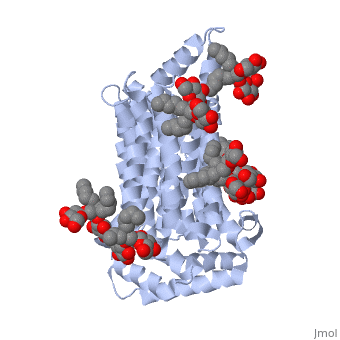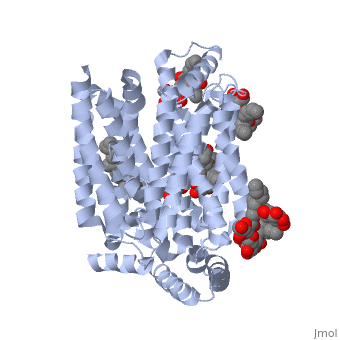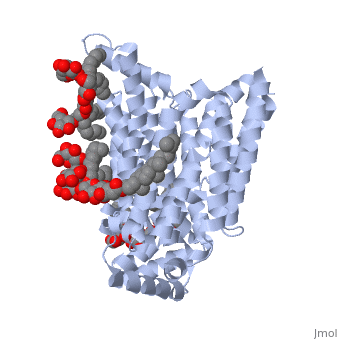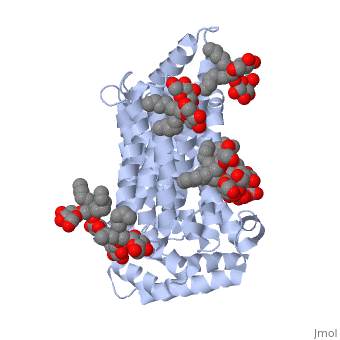
| - | GLUT3(<scene name='71/716527/5c65/1'>5c65</scene>) is a transport protein consisting of 481 amino acids and weighing 52,520 Daltons in its asymmetrical unit<ref name="eighteen">http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/</ref>. This protein is an alpha-helical protein consisting of two chains, two different ligands and water. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.65 Angstroms. GLUT3 consists of 12 transmembrane segments (TMs) folded “into the N-terminal and C-terminal domains, each comprising ‘3+3’ inverted repeats”<ref name="nine"/> These TMs consist of four 3 repeated sections. [http://www.nature.com/nature/journal/v526/n7573/images_article/nature14655-f1.jpg Here] is a figure by Deng, D., et al. showing these repeated transmembrane segments<ref name="nine">Deng, D., Sun, P., Yan, C., Ke, M., Jiang, X., Xiong, L., . . . Yan, N. (2015). Molecular basis of ligand recognition and transport by glucose transporters. Nature, 526(7573), 391-396. doi:10.1038/nature14655</ref>. The protein consists of two different ligands, Y01(green) and 37X(green). Octyl Glucose Neopentyl Glycol (<scene name='pdbligand=37X:OCTYL+GLUCOSE+NEOPENTYL+GLYCOL'>37X</scene>) has a chemical formula of C27H52O12 and a molecular weight of 569 Da. There are six 37X (501-506a) bound to chain A of 5c65. These ligands are kept in place by hydrogen bonds to arginine, proline, and serine and by van der Waals forces. Chain B has three 37X ligands attached to it (501-503b). These are attached through hydrogen bonds by arginine, proline, and serine as well as by van der Waals forces. To view 37X in 3D use [http://www.rcsb.org/pdb/explore/jmol.do?structureId=5C65&residueNr=37X | + | GLUT3(<scene name='71/716527/5c65/1'>5c65</scene>) is a transport protein consisting of 481 amino acids and weighing 52,520 Daltons in its asymmetrical unit<ref name="eighteen">http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/</ref>. This protein is an alpha-helical protein consisting of two chains, two different ligands and water. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.65 Angstroms. GLUT3 consists of 12 transmembrane segments (TMs) folded “into the N-terminal and C-terminal domains, each comprising ‘3+3’ inverted repeats”<ref name="nine"/> These TMs consist of four 3 repeated sections. [http://www.nature.com/nature/journal/v526/n7573/images_article/nature14655-f1.jpg Here] is a figure by Deng, D., et al. showing these repeated transmembrane segments<ref name="nine">Deng, D., Sun, P., Yan, C., Ke, M., Jiang, X., Xiong, L., . . . Yan, N. (2015). Molecular basis of ligand recognition and transport by glucose transporters. Nature, 526(7573), 391-396. doi:10.1038/nature14655</ref>. The protein consists of two different ligands, Y01(green) and 37X(green). Octyl Glucose Neopentyl Glycol (<scene name='pdbligand=37X:OCTYL+GLUCOSE+NEOPENTYL+GLYCOL'>37X</scene>) has a chemical formula of C27H52O12 and a molecular weight of 569 Da. There are six 37X (501-506a) bound to chain A of 5c65. These ligands are kept in place by hydrogen bonds to arginine, proline, and serine and by van der Waals forces. Chain B has three 37X ligands attached to it (501-503b). These are attached through hydrogen bonds by arginine, proline, and serine as well as by van der Waals forces. To view 37X in 3D use [http://www.rcsb.org/pdb/explore/jmol.do?structureId=5C65&residueNr=37X JSmol]. Cholesterol hemisuccinate (<scene name='pdbligand=Y01:CHOLESTEROL+HEMISUCCINATE'>Y01</scene>) has a chemical formula of C31H50O4 and has a molecular weight of 487 Da. One Y01 is attached to chain a and another Y01 is attached to chain b. To view Y01 in 3D use [http://www.rcsb.org/pdb/explore/jmol.do?structureId=5C65&residueNr=Y01 JSmol]. GLUT3 was also identified and analyzed in a complex with alpha & beta d-glucose. This model was reported with a resolution of 1.5 Å and was in an open-occluded state. The alpha and beta d glucose were coordinated by amino acids N315, E378, Q159, W368, Q280, Q281, N286. These are located on TM8 and TM10a and TM10b. GLUT3 structure was also determined when bound to maltose in an outward-open and an outward-occluded conformation. This was measure to a resolution of 2.6 Å and 2.4 Å respectively. (include Figure 3 part a). To get a better view of the structure of the protein use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=5C65 FirstGlance]. |
This is 5c65 shown through <scene name="/12/3456/Sample/1">colored groups</scene>. This is 5c65 shown through a <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. | This is 5c65 shown through <scene name="/12/3456/Sample/1">colored groups</scene>. This is 5c65 shown through a <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. | ||
Revision as of 05:10, 17 November 2015
Facilitated Glucose Transporter 3, Solute Carrier Family 2 (GLUT3/ SLC2A3) in Homo Sapiens
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Long, W., & Cheeseman, C. I. (2015). Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health and Cytoskeleton, 7, 167-183. doi:10.2147/CHC.S60484
- ↑ Kipmen-Korgun, D., Bilmen-Sarikcioglu, S., Altunbas, H., Demir, R., & Korgun, E. T. (2009). Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes.Scandinavian Journal of Clinical and Laboratory Investigation, 69(3), 350-358. doi:10.1080/00365510802632163
- ↑ 3.0 3.1 Simpson,I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., & Vannucci, S. J. (2008). The facilitative glucose transporter GLUT3: 20 years of distinction. American Journal of Physiology - Endocrinology and Metabolism, 295(2), E242-E253. doi:10.1152/ajpendo.90388.2008
- ↑ Maher, F., Vannucci, S. J., & Simpson, I. A. (1994). Glucose transporter proteins in brain. FASEB Journal, 8(13), 1003-1011.
- ↑ Xu, J., Lu, C., Wang, J., Zhang, R., Qian, X., & Zhu, H. (2015). Regulation of human trophoblast GLUT3 glucose transporter by mammalian target of rapamycin signaling. International Journal of Molecular Sciences, 16(6), 13815-13828. doi:10.3390/ijms160613815
- ↑ Liu, Y., Liu, F., Iqbal, K., Grundke-Iqbal, I., & Gong, C. -. (2008). Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in alzheimer disease. FEBS Letters, 582(2), 359-364. doi:10.1016/j.febslet.2007.12.035
- ↑ http://www.ebi.ac.uk/pdbe/entry/pdb/5c65/
- ↑ 8.0 8.1 Deng, D., Sun, P., Yan, C., Ke, M., Jiang, X., Xiong, L., . . . Yan, N. (2015). Molecular basis of ligand recognition and transport by glucose transporters. Nature, 526(7573), 391-396. doi:10.1038/nature14655
- ↑ 9.0 9.1 Naftalin RJ, Holman GD. Transport of sugars in human red cells. In: Ellory JC, Lew V, editors. \ Membrane Transport in Red Cells. New York, NY, USA: Academic Press; 1977.
- ↑ 10.0 10.1 Carruthers, A., DeZutter, J., Ganguly, A., & Devaskar, S. U. (2009). Will the original glucose transporter isoform please stand up! American Journal of Physiology - Endocrinology and Metabolism, 297(4), E836-E848. doi:10.1152/ajpendo.00496.2009
- ↑ Jardetzky, O. (1966). Simple allosteric model for membrane pumps [27]. Nature, 211(5052), 969-970. doi:10.1038/211969a0
- ↑ Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615.
- ↑ Caulfield MJ, Munroe PB, O’Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:1509–1523.
- ↑ Vollers, S. S., & Carruthers, A. (2012). Sequence determinants of GLUT1-mediated accelerated-exchange transport: Analysis by homology-scanning mutagenesis. Journal of Biological Chemistry, 287(51), 42533-42544.doi:10.1074/jbc.M112.369587