Butyrylcholinesterase
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
== Structural highlights == | == Structural highlights == | ||
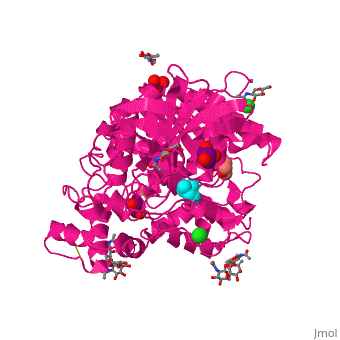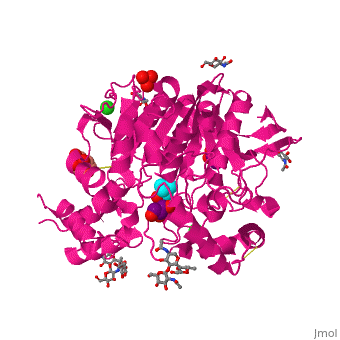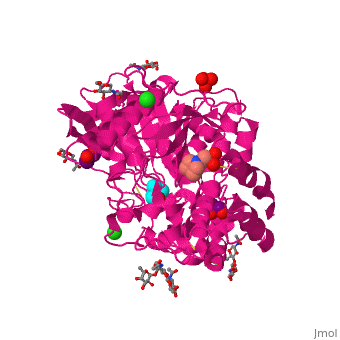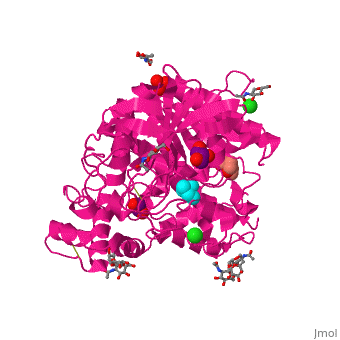
| - | Like in the AChE structure, BChE active site is located at the bottom of a ca. 20A deep gorge. The <scene name='39/399020/Cv/4'>active site of BChE</scene> is similar to that of AChE. | + | Like in the AChE structure, BChE active site is located at the bottom of a ca. 20A deep gorge. The <scene name='39/399020/Cv/4'>active site of BChE</scene> is similar to that of AChE. <ref>PMID:12869558</ref> The differences are noticed in the lining of the gorge were some of the aromatic residues in AChE are substituted by hydrophobic ones and in the active site acyl-binding pocket where 2 Phe residues are replaced by Leu and Val in BChE. |
</StructureSection> | </StructureSection> | ||
| Line 63: | Line 63: | ||
For additional information, see: [[Alzheimer's Disease]] | For additional information, see: [[Alzheimer's Disease]] | ||
<br /> | <br /> | ||
| - | + | == References == | |
| + | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 12:59, 17 November 2015
| |||||||||||
3D structures of BChE
Updated on 17-November-2015
Additional Resources
For additional information, see: Alzheimer's Disease
References
- ↑ Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003 Oct 17;278(42):41141-7. Epub 2003 Jul 17. PMID:12869558 doi:http://dx.doi.org/10.1074/jbc.M210241200
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Lakshmi Venkatachalam, Joel L. Sussman, Jaime Prilusky, David Canner