User:Michael Adams/Sandbox 1
From Proteopedia
| Line 5: | Line 5: | ||
In Strong and Ellington’s 1994 experiment, arginine kinase (AK) was isolated from Limulus polyphemus, the Atlantic horseshoe crab, a marine chelicerate arthropod (Strong and Ellington, 1994). They isolated the gene for AK, sequenced the DNA and identified it to be 1071 nucleotides. The 1071 nucleotides produce a 357 amino acid protein and AK extracted from other organisms show similarity to this protein. AK serves a similar function to that of creatine kinase, in vertebrates (Strong and Ellington, 1994). The enzyme creatine kinase maintains energy homeostasis by producing ATP in high energy requiring cells such as skeletal and cardiac muscle and neurons (Wallimann et al, 1998). This is done by the transfer of an N-phosphoryl group from phosphocreatine to ADP. | In Strong and Ellington’s 1994 experiment, arginine kinase (AK) was isolated from Limulus polyphemus, the Atlantic horseshoe crab, a marine chelicerate arthropod (Strong and Ellington, 1994). They isolated the gene for AK, sequenced the DNA and identified it to be 1071 nucleotides. The 1071 nucleotides produce a 357 amino acid protein and AK extracted from other organisms show similarity to this protein. AK serves a similar function to that of creatine kinase, in vertebrates (Strong and Ellington, 1994). The enzyme creatine kinase maintains energy homeostasis by producing ATP in high energy requiring cells such as skeletal and cardiac muscle and neurons (Wallimann et al, 1998). This is done by the transfer of an N-phosphoryl group from phosphocreatine to ADP. | ||
== Structure == | == Structure == | ||
| - | The structure of arginine kinase is mainly α-helical and contains an N-terminal region with a specificity loop for specific substrate binding. (Figure 1b). However, when compared to creatine kinase, arginine kinase is not terminated at the N-terminal end with a pair of proline-glycine residues. Typically within creatine kinase, the proline molecules restrict changes in conformation and is the amino acid that terminates helices. The glycine chains are usually associated with flexibility. However, in arginine kinase this is typically not the case. On the C-terminal end, there is an eight-stranded antiparallel β-sheet with seven α-helices flanking the sheet (Figure 1). [[Image:F1.large | + | The structure of arginine kinase is mainly α-helical and contains an N-terminal region with a specificity loop for specific substrate binding. (Figure 1b). However, when compared to creatine kinase, arginine kinase is not terminated at the N-terminal end with a pair of proline-glycine residues. Typically within creatine kinase, the proline molecules restrict changes in conformation and is the amino acid that terminates helices. The glycine chains are usually associated with flexibility. However, in arginine kinase this is typically not the case. On the C-terminal end, there is an eight-stranded antiparallel β-sheet with seven α-helices flanking the sheet (Figure 1). [[Image:F1.large.jpg]] |
The small domain specificity loop forms a “specificity” pocket surrounding the methyl substituent of the guanidinium group that is unique to creatine substrates. In this region, five residues differ between arginine and creatine kinases: 312, 314, 315, 317, and 319 (Newsholme et al, 1978). Within each arginine kinase, there is typically a Mg+2 ion adjacent to the antiparallel β-sheet (Figure 1b). Typically two arginine kinase structures mirror each other and form a hole like structure in between the two. However, when a substrate is in the binding site, the active site remains unchanged and does not change in conformation (Figure 1a). | The small domain specificity loop forms a “specificity” pocket surrounding the methyl substituent of the guanidinium group that is unique to creatine substrates. In this region, five residues differ between arginine and creatine kinases: 312, 314, 315, 317, and 319 (Newsholme et al, 1978). Within each arginine kinase, there is typically a Mg+2 ion adjacent to the antiparallel β-sheet (Figure 1b). Typically two arginine kinase structures mirror each other and form a hole like structure in between the two. However, when a substrate is in the binding site, the active site remains unchanged and does not change in conformation (Figure 1a). | ||
Revision as of 23:48, 7 December 2015
|
Contents |
Arginine Kinase
A phosphokinase used to store energy in the form of Argininephosphate.
Discovery and Isolation
In Strong and Ellington’s 1994 experiment, arginine kinase (AK) was isolated from Limulus polyphemus, the Atlantic horseshoe crab, a marine chelicerate arthropod (Strong and Ellington, 1994). They isolated the gene for AK, sequenced the DNA and identified it to be 1071 nucleotides. The 1071 nucleotides produce a 357 amino acid protein and AK extracted from other organisms show similarity to this protein. AK serves a similar function to that of creatine kinase, in vertebrates (Strong and Ellington, 1994). The enzyme creatine kinase maintains energy homeostasis by producing ATP in high energy requiring cells such as skeletal and cardiac muscle and neurons (Wallimann et al, 1998). This is done by the transfer of an N-phosphoryl group from phosphocreatine to ADP.
Structure
The structure of arginine kinase is mainly α-helical and contains an N-terminal region with a specificity loop for specific substrate binding. (Figure 1b). However, when compared to creatine kinase, arginine kinase is not terminated at the N-terminal end with a pair of proline-glycine residues. Typically within creatine kinase, the proline molecules restrict changes in conformation and is the amino acid that terminates helices. The glycine chains are usually associated with flexibility. However, in arginine kinase this is typically not the case. On the C-terminal end, there is an eight-stranded antiparallel β-sheet with seven α-helices flanking the sheet (Figure 1). 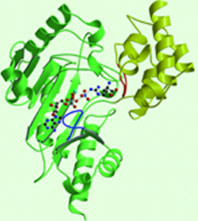
The small domain specificity loop forms a “specificity” pocket surrounding the methyl substituent of the guanidinium group that is unique to creatine substrates. In this region, five residues differ between arginine and creatine kinases: 312, 314, 315, 317, and 319 (Newsholme et al, 1978). Within each arginine kinase, there is typically a Mg+2 ion adjacent to the antiparallel β-sheet (Figure 1b). Typically two arginine kinase structures mirror each other and form a hole like structure in between the two. However, when a substrate is in the binding site, the active site remains unchanged and does not change in conformation (Figure 1a).
Function
Arginine Kinase is part of a class of kinases that regulates ATP levels in the body to help maintain homeostasis. Arginine Kinase is a phosphokinase - a kinase used in the catalyzation of phosphagens and adenosine diphosphate (ADP) into adenosine triphosphate (ATP). It creates a sort of storage option for energy. Phosphagens act as a storage form of phosphate (Nω-phospho-L-arginine) that can be catalyzed into an energy source (ATP) when needed (Pereira et al, 2000). The arginine kinase is a lock and key catalyst that holds ADP and phosphoarginine in place and catalyzes the transfer of phosphate from phosphoarginine to ADP forming arginine and ATP (Azzi et al., 2004). It is the most common phosphokinase (PK) in invertebrates. The most common PK in vertebrates is Creatine Kinase (Pereira et al, 2000).
Application to the Animal Kingdom
Arginine Kinase is the individual phosphagen kinase that is found in major invertebrates, such as: arthropods, mollusks, and echinoderms. Most recently, an arginine kinase was purified from a house fly (Walliman et al. 1973). This gave Wallimann and Eppenberger the initiative to investigate the arginine kinase in Drosophila melanogaster, also known as a fruit fly (Walliman et al. 1973). Since the genome and genetic development of Drosophila melanogaster is well understood, this allows for any discoveries made to be easily interpreted. Additionally, further discoveries will help better understand the characteristics of arginine kinase corresponding vertebrate enzyme, creatine kinase (Wallimann et al., 1973)
Arginine Kinase (AK) is represented by a single gene and the sequence or partial sequence is available from Drosophila, Limulus, lobster, shrimp, and abalone (Wang et al., 1998). In the study by Wang et al. 1998, a phylogenetic tree was put together for the evolution of Arginine Kinase compared to Creatine Kinase. It indicates that there are major clusters corresponding to Creatine Kinase and Arginine Kinase. Also, it is expressed in the gills of two species of euryhaline crabs, the blue crab Callinectes sapidus and the shore crab Carcinus maenus, in which energy-requiring functions include monovalent ion transport, acid-base balance, nitrogen excretion (Kotylar et al. 2000).