Sandbox porins
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Energetics == | == Energetics == | ||
The components and functions of porins can be brought together with its energetics to explain porins main function, simple diffusion. Porins have passive-mediated transport, which is also called facilitated diffusion. Facilitated diffusion allows sugars, ions, amino acids, and polar molecules to pass through the outer membrane. This occurs because of the result of a concentration gradient where solutes travel from an area of high concentration to an area of low concentration. More specifically, porins are channel-forming ionophores which are solvent-filled channels where selected ions can pass through. The selectivity comes from the electrochemical gradient that is formed inside the middle of the porin at the constriction zone. The constriction zone is the eyelet and is surrounded by the positively charged and negatively charged amino acids attached to opposite walls of the beta barrel and the calcium ions. The source of energy for porins and its facilitated diffusion is through its exergonic process. The driving force of this energy is from the concentration gradient, which means no energy is given by transporters. When solutes diffuse through porins from high concentration to low concentration, the entropy becomes positive and creates more disorder and free energy becomes negative. As a result, energy is released. | The components and functions of porins can be brought together with its energetics to explain porins main function, simple diffusion. Porins have passive-mediated transport, which is also called facilitated diffusion. Facilitated diffusion allows sugars, ions, amino acids, and polar molecules to pass through the outer membrane. This occurs because of the result of a concentration gradient where solutes travel from an area of high concentration to an area of low concentration. More specifically, porins are channel-forming ionophores which are solvent-filled channels where selected ions can pass through. The selectivity comes from the electrochemical gradient that is formed inside the middle of the porin at the constriction zone. The constriction zone is the eyelet and is surrounded by the positively charged and negatively charged amino acids attached to opposite walls of the beta barrel and the calcium ions. The source of energy for porins and its facilitated diffusion is through its exergonic process. The driving force of this energy is from the concentration gradient, which means no energy is given by transporters. When solutes diffuse through porins from high concentration to low concentration, the entropy becomes positive and creates more disorder and free energy becomes negative. As a result, energy is released. | ||
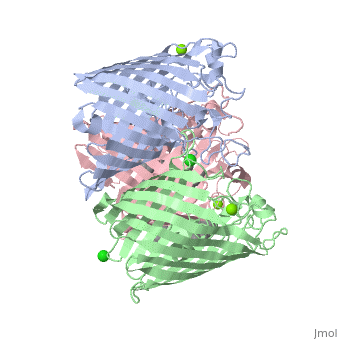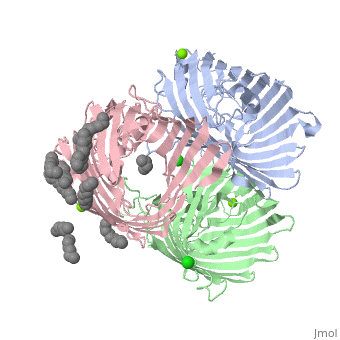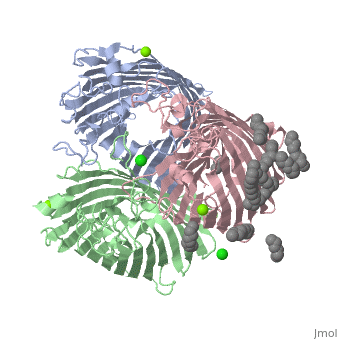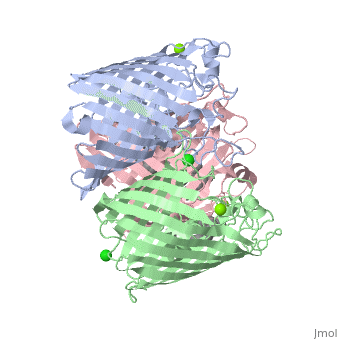
| + | ==OmpF:4gcp== | ||
| + | Non-specific | ||
| + | Contains a ligand, ampicillin (Amp): Zwitterionic (+/-) antibiotic. | ||
| + | Will allow molecules of various charges to transfer through. | ||
| + | Contains 2 chains (A,B). [[Image:4gcp.png | thumb | this is my caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==PhoE:1pho== | ||
| + | Transports phosphate compounds. | ||
| + | Contains no ligands. | ||
| + | Contains 1 chain (A). | ||
| + | Has a loop inside that is the constriction zone. | ||
| + | Creates ion selectivity for molecules to pass through. | ||
| + | Anion selective (only slightly) [[Image:1pho.png | thumb | this is my caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
== Structural highlights == | == Structural highlights == | ||
Revision as of 05:17, 9 December 2015
This page is setup for Matt to build his senior project for OU CHEM 4923
Porins Overview
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644