This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1aon
From Proteopedia
| Line 4: | Line 4: | ||
|PDB= 1aon |SIZE=350|CAPTION= <scene name='initialview01'>1aon</scene>, resolution 3.0Å | |PDB= 1aon |SIZE=350|CAPTION= <scene name='initialview01'>1aon</scene>, resolution 3.0Å | ||
|SITE= | |SITE= | ||
| - | |LIGAND= <scene name='pdbligand= | + | |LIGAND= <scene name='pdbligand=ADP:ADENOSINE-5'-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene> |
|ACTIVITY= | |ACTIVITY= | ||
|GENE= GROE ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=562 Escherichia coli]) | |GENE= GROE ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=562 Escherichia coli]) | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1aon FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1aon OCA], [http://www.ebi.ac.uk/pdbsum/1aon PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1aon RCSB]</span> | ||
}} | }} | ||
| Line 26: | Line 29: | ||
[[Category: Sigler, P B.]] | [[Category: Sigler, P B.]] | ||
[[Category: Xu, Z.]] | [[Category: Xu, Z.]] | ||
| - | [[Category: ADP]] | ||
| - | [[Category: MG]] | ||
[[Category: chaperonin assisted protein folding]] | [[Category: chaperonin assisted protein folding]] | ||
[[Category: complex (groel/groes)]] | [[Category: complex (groel/groes)]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 18:44:31 2008'' |
Revision as of 15:44, 30 March 2008
| |||||||
| , resolution 3.0Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||
| Gene: | GROE (Escherichia coli) | ||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
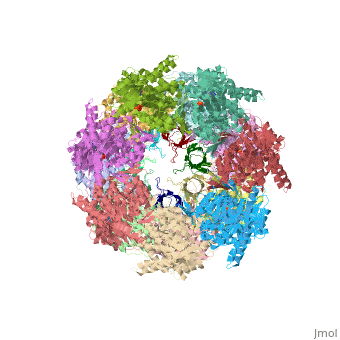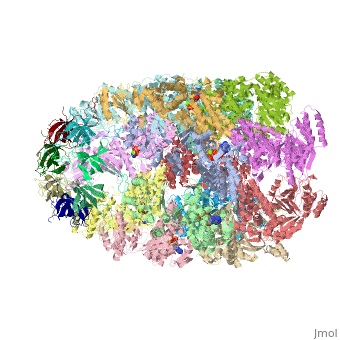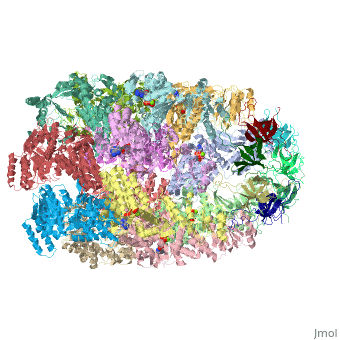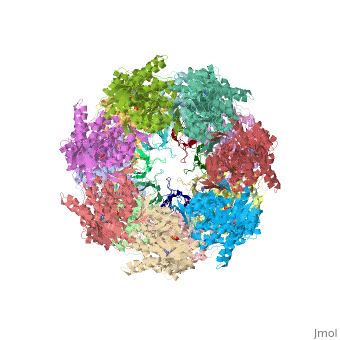
CRYSTAL STRUCTURE OF THE ASYMMETRIC CHAPERONIN COMPLEX GROEL/GROES/(ADP)7
Overview
Chaperonins assist protein folding with the consumption of ATP. They exist as multi-subunit protein assemblies comprising rings of subunits stacked back to back. In Escherichia coli, asymmetric intermediates of GroEL are formed with the co-chaperonin GroES and nucleotides bound only to one of the seven-subunit rings (the cis ring) and not to the opposing ring (the trans ring). The structure of the GroEL-GroES-(ADP)7 complex reveals how large en bloc movements of the cis ring's intermediate and apical domains enable bound GroES to stabilize a folding chamber with ADP confined to the cis ring. Elevation and twist of the apical domains double the volume of the central cavity and bury hydrophobic peptide-binding residues in the interface with GroES, as well as between GroEL subunits, leaving a hydrophilic cavity lining that is conducive to protein folding. An inward tilt of the cis equatorial domain causes an outward tilt in the trans ring that opposes the binding of a second GroES. When combined with new functional results, this negative allosteric mechanism suggests a model for an ATP-driven folding cycle that requires a double toroid.
About this Structure
1AON is a Protein complex structure of sequences from Escherichia coli. The following page contains interesting information on the relation of 1AON with [Chaperones]. Full crystallographic information is available from OCA.
Reference
The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex., Xu Z, Horwich AL, Sigler PB, Nature. 1997 Aug 21;388(6644):741-50. PMID:9285585
Page seeded by OCA on Sun Mar 30 18:44:31 2008