We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User talk:Eman AlaliSandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
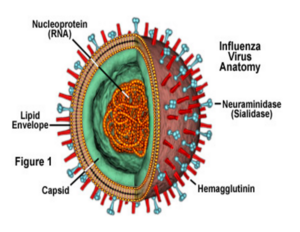
The H1 subunit consist of 328 amino acid composing eight stranded beta-sheet associated with little alpha-helix (Proteopedia, 2015). The <scene name='SAndbox_159/Ha1/1'>H1</scene> subunit forms a globular bulb at the top of the structure and contains the sialic acid binding site. The amino acid of the alpha helix and some other beta sheets compose the binding pocket of the sialic acid subunit, and determine the attachment specificity of the virus to the host cell. | The H1 subunit consist of 328 amino acid composing eight stranded beta-sheet associated with little alpha-helix (Proteopedia, 2015). The <scene name='SAndbox_159/Ha1/1'>H1</scene> subunit forms a globular bulb at the top of the structure and contains the sialic acid binding site. The amino acid of the alpha helix and some other beta sheets compose the binding pocket of the sialic acid subunit, and determine the attachment specificity of the virus to the host cell. | ||
The <scene name='SAndbox_159/Ha2/2'>H2</scene> subunit of H0 is called membrane-spanning anchor and it is directly involved in the fusion mechanism. <scene name='SAndbox_159/Ha2/2'>H2</scene> has a hairpin structure composed by two antiparallel alpha-helices. The C- terminal of <scene name='SAndbox_159/Ha2/2'>H2</scene> is embedded in the viral membrane while the N-terminal end contains 10 hydrophobic amino acid forming the fusion peptide (Proteopedia, 2011). | The <scene name='SAndbox_159/Ha2/2'>H2</scene> subunit of H0 is called membrane-spanning anchor and it is directly involved in the fusion mechanism. <scene name='SAndbox_159/Ha2/2'>H2</scene> has a hairpin structure composed by two antiparallel alpha-helices. The C- terminal of <scene name='SAndbox_159/Ha2/2'>H2</scene> is embedded in the viral membrane while the N-terminal end contains 10 hydrophobic amino acid forming the fusion peptide (Proteopedia, 2011). | ||
| - | + | [[Image:A_closer_look_on_the_structure_of_H1_and_H2_subunits_of_Hemagglutinin_protein..PNG|300px|thumb]] | |
| + | {{Clear}} | ||
When H protein attaches to sialic acid residues of the ciliated columnar epithelial cell lining the sinuses and airways, the bound virus is endocytosed by the cell and the virions enter the cell within the endosomal vesicle. Then the endosomal vesicle pH level drops and reaches about 5.5 due to the pumping of protons inside the vesicle by the M2 ions channel. As a result, the <scene name='SAndbox_159/Ha1/1'>H1</scene> subuint shifts its position, allowing the <scene name='SAndbox_159/Ha2/2'>H2</scene> subunit to become embedded in the host cell membrane. Additionally, the C-terminus embedded in the viral membrane rearranges, bringing the two membranes closer together and facilitation fusion (Wilson et. al 1981). The pH induced conformational changes are partially but not completely reversible. As the pH decrease to 5.5, the three globular heads start to dissociate due to the protonation of relevant H protein residues. the change in pH facilitate the releasing of the fusion peptides. Also at low pH the short and long alpha-helix are separated(Thoennes et.al 2008). The shifting of <scene name='SAndbox_159/Ha1/1'>H1</scene> subunit plays an important role in H mediated membrane fusion , <scene name='SAndbox_159/Ha1/1'>H1</scene> protein helps the membrane attachment and fusion, which results in the release of viral RNA’s into the cytoplasm and then into the cell’s nucleus for RNA replication (Racanilllo, 2009). | When H protein attaches to sialic acid residues of the ciliated columnar epithelial cell lining the sinuses and airways, the bound virus is endocytosed by the cell and the virions enter the cell within the endosomal vesicle. Then the endosomal vesicle pH level drops and reaches about 5.5 due to the pumping of protons inside the vesicle by the M2 ions channel. As a result, the <scene name='SAndbox_159/Ha1/1'>H1</scene> subuint shifts its position, allowing the <scene name='SAndbox_159/Ha2/2'>H2</scene> subunit to become embedded in the host cell membrane. Additionally, the C-terminus embedded in the viral membrane rearranges, bringing the two membranes closer together and facilitation fusion (Wilson et. al 1981). The pH induced conformational changes are partially but not completely reversible. As the pH decrease to 5.5, the three globular heads start to dissociate due to the protonation of relevant H protein residues. the change in pH facilitate the releasing of the fusion peptides. Also at low pH the short and long alpha-helix are separated(Thoennes et.al 2008). The shifting of <scene name='SAndbox_159/Ha1/1'>H1</scene> subunit plays an important role in H mediated membrane fusion , <scene name='SAndbox_159/Ha1/1'>H1</scene> protein helps the membrane attachment and fusion, which results in the release of viral RNA’s into the cytoplasm and then into the cell’s nucleus for RNA replication (Racanilllo, 2009). | ||
| - | + | ||
| - | + | ||
== The Neuraminidase (N) protien == | == The Neuraminidase (N) protien == | ||
Revision as of 21:45, 9 December 2015
Influenza virus Glycoproteins
| |||||||||||