User:Elisha Lacey/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='4flp' size='340' side='right' caption='Bromodomain testis specific as functional dimer=''> | <StructureSection load='4flp' size='340' side='right' caption='Bromodomain testis specific as functional dimer=''> | ||
The human genome has 56 bromodomains across 42 proteins. Bromodomains are found in mammals. Bromodomain testis specific(BRDT) and expressed in testis, spermatocytes, spermatids, and spermatozoa. BRDT is also known as known as CT9 and RING3-like protein and belongs to the bromodomain extra terminal (BET) family. BRDT is expressed in pachytene and diplotene spermatocytes and round spermatids. BRDTis not in testis subjects with sertoli cell only syndrome. | The human genome has 56 bromodomains across 42 proteins. Bromodomains are found in mammals. Bromodomain testis specific(BRDT) and expressed in testis, spermatocytes, spermatids, and spermatozoa. BRDT is also known as known as CT9 and RING3-like protein and belongs to the bromodomain extra terminal (BET) family. BRDT is expressed in pachytene and diplotene spermatocytes and round spermatids. BRDTis not in testis subjects with sertoli cell only syndrome. | ||
| - | + | <scene name='71/719804/Brdt_bromo_domains/1'>BRDT dimers</scene> | |
== Function == | == Function == | ||
| - | Bromodomain testis specific is a monomer that is only fully functional as a dimer. BRDT is a transcriptional regulator for gene expression during spermatogenesis. | + | Bromodomain testis specific is a monomer that is only fully functional as a dimer. BRDT is a transcriptional regulator for gene expression during spermatogenesis. BRDT is important to healthy progeny, without it sperm is unable to correctly compact genome. BRDT gives rise to the shape of sperm cells, without it the sperm cells are misshapen. |
== BRDT and fertility == | == BRDT and fertility == | ||
Loss of one of the bromodomains or even single point mutations leads to decrease spermatogenesis and defective sperm. If both bromodomains are knocked out it leads to loss of fertility. | Loss of one of the bromodomains or even single point mutations leads to decrease spermatogenesis and defective sperm. If both bromodomains are knocked out it leads to loss of fertility. | ||
== Relevance == | == Relevance == | ||
| - | Male contraception has been eluding scientists for years and is a desired area to prevent unplanned pregnancies | + | Male contraception has been eluding scientists for years and is a desired area to prevent unplanned pregnancies. In studies of an anti-cancer drug triazolotheinodiaepine known as JQ1 that inhibits BRD4 in cancer pathogenesis. In experiments in mice it is seen, due to structural similarities, JQ1 blocks the production of sperm in testes. The binding of JQ1 in the acetyl-lysine pocket prevents recognition of acetylated histone H4KAC4. JQ1 in studies is not seen to affect hormone levels(leutinizing hormone. follicle stimulating hormone, testosterone), mating behaviors or harmful effects to eventual offspring. Also JQ1 is not seen to cause sterility in mice. The effects of JQ1 on fertility are reversible and dose and time dependent effects on fertility. In mice it reduces size of testis, seminiferous tubes, and number of spermatozoa as well as the mobility of spermatozoa. |
[[Image:JQ1 strucutre.jpg]] | [[Image:JQ1 strucutre.jpg]] | ||
==Analysis of Structure == | ==Analysis of Structure == | ||
| - | The functional unit for BRDT is two bromodomain motifs, each | + | The functional unit for BRDT is two bromodomain motifs, each monomer is composed of 4 anti-parallel alpha helixes. At the end of the helix bundles is the acetylated-lysine binding site binding site. BRDT belongs to the histone acetyltransferase superfamily. BRDT is tissue restricted to the testis. BRDT activates the histones by hyper acetylation at the promoter of genes leading to condensed acetylated chromatin of a haploid spermatid. Running an alignment gives 200 sequence similarities which shows that this sequence is highly conserved among organism in the phylogenic tree. |
| - | + | ||
==Analysis of related Sequences== | ==Analysis of related Sequences== | ||
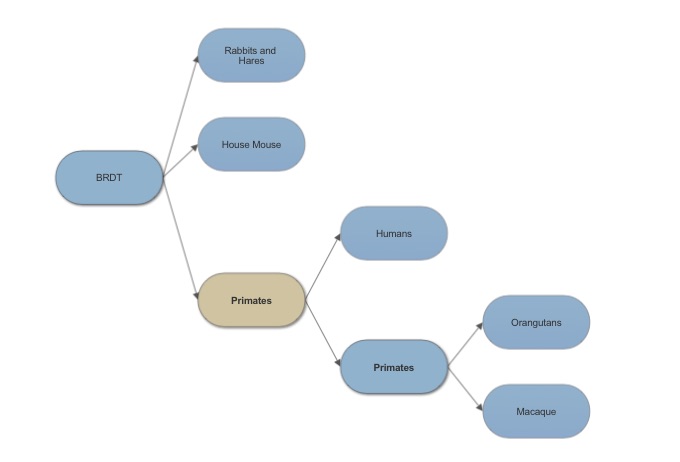
| - | Bromodomain testis specific shares 97% sequence | + | Bromodomain testis specific in humans shares 97% sequence identity with bromodomain testis specific in Sumatran Orangutans. These genes are close orthologs with such similar sequence identity and they both serve a function in fertility. |
==Evolution of BRDT== | ==Evolution of BRDT== | ||
[[Image:BRDT tree.jpg]] | [[Image:BRDT tree.jpg]] | ||
Revision as of 11:19, 10 December 2015
Bromodomain testis specific
| |||||||||||
References
Berkovits B, Wolgemuth D. The first bromodomain of the testis-specific double bromodomain protein Brdt is required for chromocenter organization that is modulated by genetic background. PMC. 2011 Dec 15: 360 (2):358-368.
Barda S, Yogev L, Paz G, et al. BRDT gene sequence in human testicular pathologies and the implication of its single nucleotide polymorphism (rs3088232) on fertility. Andrology. 2014 May 28; 2(4): 641-647.
Zdrojewicz Z, Konieczyn R, Papier P, Szten F.Brdt Bromodomains Inhibitors and Other Modern Means of Male Contraception. ACEM. 2015 Feb 23; 24(4):705-714.