This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Elisha Lacey/Sandbox 1
From Proteopedia
| Line 2: | Line 2: | ||
<StructureSection load='4flp' size='340' side='right' caption='Bromodomain testis specific as functional dimer=''> | <StructureSection load='4flp' size='340' side='right' caption='Bromodomain testis specific as functional dimer=''> | ||
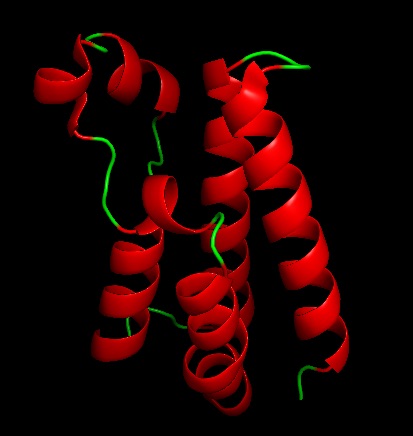
The human genome has 56 bromodomains across 42 proteins. Bromodomains are found in mammals. Bromodomain testis specific(BRDT) and expressed in testis, spermatocytes, spermatids, and spermatozoa. BRDT is also known as known as CT9 and RING3-like protein and belongs to the bromodomain extra terminal (BET) family. BRDT is expressed in pachytene and diplotene spermatocytes and round spermatids. BRDTis not in testis subjects with sertoli cell only syndrome. | The human genome has 56 bromodomains across 42 proteins. Bromodomains are found in mammals. Bromodomain testis specific(BRDT) and expressed in testis, spermatocytes, spermatids, and spermatozoa. BRDT is also known as known as CT9 and RING3-like protein and belongs to the bromodomain extra terminal (BET) family. BRDT is expressed in pachytene and diplotene spermatocytes and round spermatids. BRDTis not in testis subjects with sertoli cell only syndrome. | ||
| + | [[Image:Simple diagram brdt.jpg]] | ||
<scene name='71/719804/Brdt_bromo_domains/1'>BRDT dimers</scene> | <scene name='71/719804/Brdt_bromo_domains/1'>BRDT dimers</scene> | ||
== Function == | == Function == | ||
| Line 12: | Line 13: | ||
==Analysis of Structure == | ==Analysis of Structure == | ||
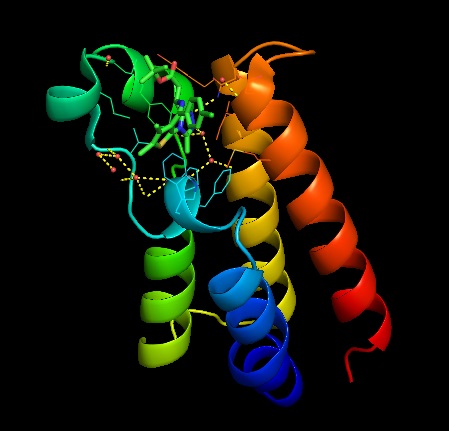
The functional unit for BRDT is two bromodomain motifs, each monomer is composed of 4 anti-parallel alpha helixes. The 2 monomers are not unique to one another. At the end of the helix bundles is the acetylated-lysine binding site binding site. BRDT belongs to the histone acetyltransferase superfamily. BRDT is tissue restricted to the testis. BRDT activates the histones by hyper acetylation at the promoter of genes leading to condensed acetylated chromatin of a haploid spermatid. Running an alignment gives 200 sequence similarities which shows that this sequence is highly conserved among organism in the phylogenic tree. This was also seen when running multiple alignments and the sequence was similar among several different organisms. | The functional unit for BRDT is two bromodomain motifs, each monomer is composed of 4 anti-parallel alpha helixes. The 2 monomers are not unique to one another. At the end of the helix bundles is the acetylated-lysine binding site binding site. BRDT belongs to the histone acetyltransferase superfamily. BRDT is tissue restricted to the testis. BRDT activates the histones by hyper acetylation at the promoter of genes leading to condensed acetylated chromatin of a haploid spermatid. Running an alignment gives 200 sequence similarities which shows that this sequence is highly conserved among organism in the phylogenic tree. This was also seen when running multiple alignments and the sequence was similar among several different organisms. | ||
| + | [[Image:JQ1 binding site.jpg]] | ||
==Analysis of related Sequences== | ==Analysis of related Sequences== | ||
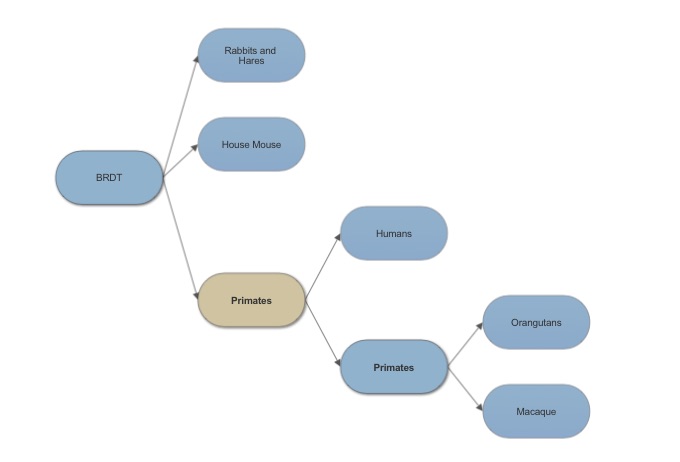
Bromodomain testis specific in humans shares 97% sequence identity with bromodomain testis specific in Sumatran Orangutans. It also shows 99% sequence similarity. These genes are close orthologs with such similar sequence identity and they both serve a function in fertility. The most distant sequence alignment is a black flying fox with and identity of 89% and still is an ortholog to BRDT in humans. Looking at an alignment comparison of BRDT and 16 other proteins the representation shows a sequence match of the alpha helixes and the non aligned segments as being random coils connecting the helices. | Bromodomain testis specific in humans shares 97% sequence identity with bromodomain testis specific in Sumatran Orangutans. It also shows 99% sequence similarity. These genes are close orthologs with such similar sequence identity and they both serve a function in fertility. The most distant sequence alignment is a black flying fox with and identity of 89% and still is an ortholog to BRDT in humans. Looking at an alignment comparison of BRDT and 16 other proteins the representation shows a sequence match of the alpha helixes and the non aligned segments as being random coils connecting the helices. | ||
[[Image:Alignmentofbrdt.jpg]] | [[Image:Alignmentofbrdt.jpg]] | ||
| + | This image above is just a short segment of aligned sequence to illustrate the sequence similar regions, going down the rows are different sources of DNA in the phylogenic tree. In red are the aligning codes and in grey are the non match sequences. The image was included to illustrate a part of the conserved sequence among orthologs. | ||
==Evolution of BRDT== | ==Evolution of BRDT== | ||
[[Image:BRDT tree.jpg]] | [[Image:BRDT tree.jpg]] | ||
| Line 25: | Line 28: | ||
Barda S, Yogev L, Paz G, et al. BRDT gene sequence in human testicular pathologies and the implication of its single nucleotide polymorphism (rs3088232) on fertility. Andrology. 2014 May 28; 2(4): 641-647. | Barda S, Yogev L, Paz G, et al. BRDT gene sequence in human testicular pathologies and the implication of its single nucleotide polymorphism (rs3088232) on fertility. Andrology. 2014 May 28; 2(4): 641-647. | ||
| + | |||
| + | Madden T. The BLAST Sequence Analysis Tool. 2002 Oct 9 [Updated 2003 Aug 13]. In: McEntyre J, Ostell J, editors. The NCBI Handbook [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2002-. Chapter 16. Available from: http://www.ncbi.nlm.nih.gov/books/NBK21097/ | ||
Zdrojewicz Z, Konieczyn R, Papier P, Szten F.Brdt Bromodomains Inhibitors and Other Modern Means of Male Contraception. ACEM. 2015 Feb 23; 24(4):705-714. | Zdrojewicz Z, Konieczyn R, Papier P, Szten F.Brdt Bromodomains Inhibitors and Other Modern Means of Male Contraception. ACEM. 2015 Feb 23; 24(4):705-714. | ||
| + | |||
| + | |||
<references/> | <references/> | ||
Revision as of 12:19, 10 December 2015
Bromodomain testis specific
| |||||||||||
References
Berkovits B, Wolgemuth D. The first bromodomain of the testis-specific double bromodomain protein Brdt is required for chromocenter organization that is modulated by genetic background. PMC. 2011 Dec 15: 360 (2):358-368.
Barda S, Yogev L, Paz G, et al. BRDT gene sequence in human testicular pathologies and the implication of its single nucleotide polymorphism (rs3088232) on fertility. Andrology. 2014 May 28; 2(4): 641-647.
Madden T. The BLAST Sequence Analysis Tool. 2002 Oct 9 [Updated 2003 Aug 13]. In: McEntyre J, Ostell J, editors. The NCBI Handbook [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2002-. Chapter 16. Available from: http://www.ncbi.nlm.nih.gov/books/NBK21097/
Zdrojewicz Z, Konieczyn R, Papier P, Szten F.Brdt Bromodomains Inhibitors and Other Modern Means of Male Contraception. ACEM. 2015 Feb 23; 24(4):705-714.