Sandbox effluxpumps
From Proteopedia
| Line 25: | Line 25: | ||
==ABCB6== | ==ABCB6== | ||
| - | ABCB6 is an ABC transport protein that is located in the outer mitochondrial membrane. This transporter is involved in multi-drug resistance and antigen presentation and is required for porphyrin uptake. | + | ABCB6 is an ABC transport protein that is located in the outer mitochondrial membrane. This transporter is involved in multi-drug resistance and antigen presentation and is required for porphyrin uptake. <ref> Krishnamurthy, P. C., Du, G., Fukuda, Y., Sun, D., Sampath, J., Mercer, K. E., Wang, J., Sosa-Pineda, B., Murti, K. G., Schuetz, J. D. Identification of a mammalian mitochondrial porphyrin transporter. Nature 443: 586-589, 2006. </ref> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 18:42, 11 December 2015
This page is setup for Angela to build her senior project for OU CHEM 4923
Contents |
Your Heading Here (maybe something like 'Structure')
|
This is a default text for your page Sandbox effluxpumps. Click above on edit this page to modify. Be careful with the < and > signs. You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Efflux pumps function to move different substances out of cells such as ions, lipids, and molecules that are toxic for the cell. [3] ATP-binding cassette (ABC) transporters are a common class of efflux pumps found in all forms of life. Humans have forty-eight known ABC transporters. An example of an ABC transporter is ABCB6.
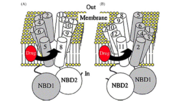
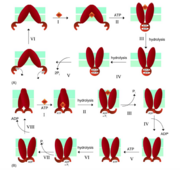
An ATP-switch model for transport has been used to describe ABC transporters. For transport, a switch between two conformations of the nucleotide binding domain dimer serves as the promoter. The binding of ATP induces the rotation of domains within the nucleotide-binding domain. A closed dimer with two molecules of ATP between the dimer is formed. The hydrolysis of ATP into ADP and Pi returns the dimer to an open configuration. The binding of ATP to the nucleotide binding domains and the closed dimer formation induces conformational changes in the transmembrane domains that aid in the movement of substrate out of the cell. The transmembrane domain is shifted so that the domain is visible to the extracellular face of the membrane and the substrates can then be released. Similar to the nucleotide-binding domain, the hydrolysis of ATP to ADP and Pi restores the dimer to its beginning conformation.
Structural Highlights
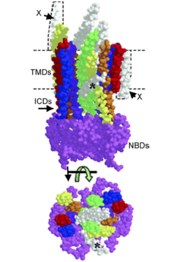
One unit of an ATP-binding cassette consists of a nucleotide binding domain and a trans-membrane domain that has six α-helices. Hydrophilic loops and the nucleotide-binding domain separate the α-helices. [3] Two nucleotide-binding domains are responsible for binding to and hydrolyzing ATP. The two transmembrane domains of a functional ABC transporter are used to form the chamber that substrates use to move across the membrane. [4] It is important to note that in ABC transporters the Walker A and B motifs are conserved and have a role in hydrogen bonding to and hydrolyzing ATP, an ABC transporter signature motif. The different motifs in ABC transporters form the ATP binding site. [3]
Energetics
There are two theories for the power stroke for ABC transporters. One model states that the formation of a “nucleotide sandwich” dimer results in changes in conformation that are relayed to the drug binding site, followed by two ATP hydrolysis events that changes the P-gp molecule to its starting conformation.[5] Another model also requires two ATP hydrolysis events, one hydrolysis event is used to drive the efflux of the drug and another hydrolysis event is used to bring the protein back to its beginning conformation.[5] Both models follow an “alternating catalytic sites” scheme in which only one of the nucleotide-binding domains hydrolyzes ATP and the two nucleotide-binding domains alternate during the catalytic cycles. [5] The energy of ATP hydrolysis is necessary to bring the protein back to its initial state.
ABCB6
ABCB6 is an ABC transport protein that is located in the outer mitochondrial membrane. This transporter is involved in multi-drug resistance and antigen presentation and is required for porphyrin uptake. [6]
</StructureSection>
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 M. Hennessy and J.P. Spiers, “A Primer on the Mechanics of P-glycoprotein the Multidrug Transporter,” Pharmacological Research 55 (2007): 1.
- ↑ Christopher F. Higgins, “Multiple Molecular Mechanisms for Multidrug Resistance Transporters,” Nature 446 (2007): 750.
- ↑ 5.0 5.1 5.2 Suresh V. Ambudkar, In-Wha Kim, and Zuben E. Sauna “The Power of the Pump: Mechanisms of Action of P-glycoprotein (ABCB1),” Science Direct 27 (2006): 393.
- ↑ Krishnamurthy, P. C., Du, G., Fukuda, Y., Sun, D., Sampath, J., Mercer, K. E., Wang, J., Sosa-Pineda, B., Murti, K. G., Schuetz, J. D. Identification of a mammalian mitochondrial porphyrin transporter. Nature 443: 586-589, 2006.