We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox flippases
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
The beginning conformation is an outward-occluded conformation and the head of Linked-lipid Oligosaccharide(LLO) is on the cytoplasmic side. When the tail of LLO is attached to external helix, the ADP exchanges to ATP and the conformation changes to outward-open state. The head of LLO attaches to a positively charged belt facing to periplasmic side since the pyrophosphate has negative charge which attracts to positive charge. As ATP changes to ADP, the conformation returns to outward-occluded conformation and it squeezes the LLO head out to periplasmic side of the membrane and the tail of LLO is released to extend to cytoplasmic side. Therefore, the position of LLO is opposite through this mechanism. <ref name= "name"> Verchère, A., & Menon, A. K. (2015). Structural biology: Lipid gymnastics. Nature, 524(7566), 420-422. </ref> | The beginning conformation is an outward-occluded conformation and the head of Linked-lipid Oligosaccharide(LLO) is on the cytoplasmic side. When the tail of LLO is attached to external helix, the ADP exchanges to ATP and the conformation changes to outward-open state. The head of LLO attaches to a positively charged belt facing to periplasmic side since the pyrophosphate has negative charge which attracts to positive charge. As ATP changes to ADP, the conformation returns to outward-occluded conformation and it squeezes the LLO head out to periplasmic side of the membrane and the tail of LLO is released to extend to cytoplasmic side. Therefore, the position of LLO is opposite through this mechanism. <ref name= "name"> Verchère, A., & Menon, A. K. (2015). Structural biology: Lipid gymnastics. Nature, 524(7566), 420-422. </ref> | ||
| + | == Energetic == | ||
| + | As the tail of LLO is attached to external helix, it acts as the switch for the ATPase to supply the ATP. <ref name= "name"> Perez, C., Gerber, S., Boilevin, J., Bucher, M., Darbre, T., Aebi, M., ... & Locher, K. P. (2015). Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524(7566), 433-438. </ref> | ||
== Disease == | == Disease == | ||
| Line 25: | Line 27: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | <ref> Perez, C., Gerber, S., Boilevin, J., Bucher, M., Darbre, T., Aebi, M., ... & Locher, K. P. (2015). Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524(7566), 433-438. </ref> | ||
<references/> | <references/> | ||
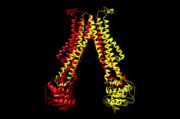
[[Image:5c76.png | thumb | this is my caption of 5c76]] | [[Image:5c76.png | thumb | this is my caption of 5c76]] | ||
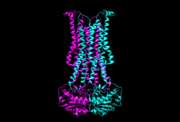
[[Image:5c73.png | thumb | this is my caption of 5c73]] | [[Image:5c73.png | thumb | this is my caption of 5c73]] | ||
Revision as of 05:47, 16 December 2015
This page is setup for Leah to build her senior project for OU CHEM 4923
Flippases
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 Verchère, A., & Menon, A. K. (2015). Structural biology: Lipid gymnastics. Nature, 524(7566), 420-422.
- ↑ Perez, C., Gerber, S., Boilevin, J., Bucher, M., Darbre, T., Aebi, M., ... & Locher, K. P. (2015). Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524(7566), 433-438.