Sandbox porins
From Proteopedia
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
| - | The general structure of porins is most recognized by its secondary structures. [[Image:PorinStructure.gif | thumb | Trimer and Monomer view of Porins <ref> “Porin Channel Protein in Escherichia coli Outer Membrane.” Rs.Noda. Web. 4 Nov. 2015. http://www.rs.noda.tus.ac.jp/~biost/OPFU/yama/public_html/study/folding/md/porin/porin.htm </ref>]] Porins secondary structures consist of a beta-barrel and most commonly have an oligomeric state as a homotrimer, which consists of three identical subunits each being a beta-barrel. The beta-barrel is normally made up of 300 to 400 amino acids, which normally forms around 16 anti-parallel beta strands, which are tilted at 45-degree angles. Each beta strand consists of an arrangement of alternating polar/non-polar amino acids. Residues of porins located at the exterior of the beta-barrel consists of non-polar hydrophobic amino acid residues, the middle consists of aromatic residues, and the interior consists of polar hydrophilic amino acid residues. The beta-barrel is also surrounded by a non-polar hydrophobic belt, which is composed of both phenylalanine and tyrosine residues. Porins additionally have pores, water-filled channels inside, beta-hairpins that make up the smooth end of the beta-barrel, and irregular loops which make up the rough end of the beta-barrel. Located in the center of porins is a constriction zone located halfway down the water-filled channel called the eyelet. The eyelet contains both positively and negative charged amino acids. Positively charged amino acids consists of, three arginine, one histidine, and two lysine, which are located on one side of the beta-barrel wall. Negatively charged amino acids consist of, four glutamic acids and seven aspartic acids located on the opposite wall the beta-barrel. To counter act the excess of negative charge, there are two calcium ions attached near the eyelet. These positively and negatively charged amino acids are located at opposite sides of the water-filled channel to create an electric field. The inside of porins, the hole of the beta-barrel is composed of hydrophilic chains. Lastly, some porins have salt-bridges between their N-terminus and C-terminus. | + | The general structure of porins is most recognized by its secondary structures. [[Image:PorinStructure.gif | thumb | Trimer and Monomer view of Porins <ref> “Porin Channel Protein in Escherichia coli Outer Membrane.” Rs.Noda. Web. 4 Nov. 2015. http://www.rs.noda.tus.ac.jp/~biost/OPFU/yama/public_html/study/folding/md/porin/porin.htm </ref>]] Porins secondary structures consist of a beta-barrel and most commonly have an oligomeric state as a homotrimer, which consists of three identical subunits each being a beta-barrel. The beta-barrel is normally made up of 300 to 400 amino acids, which normally forms around 16 anti-parallel beta strands, which are tilted at 45-degree angles<ref>“Biophysical Methods Lecture 4: Membrane Proteins – Porins.” Chembio. Department of Chemistry and Biochemistry University of Guelph. Nov. 2013. Web. 4 Nov. 2015. http://www.chembio.uoguelph.ca/educmat/phy456/456lec04.htm</ref>. Each beta strand consists of an arrangement of alternating polar/non-polar amino acids. Residues of porins located at the exterior of the beta-barrel consists of non-polar hydrophobic amino acid residues, the middle consists of aromatic residues, and the interior consists of polar hydrophilic amino acid residues. The beta-barrel is also surrounded by a non-polar hydrophobic belt, which is composed of both phenylalanine and tyrosine residues. Porins additionally have pores, water-filled channels inside, beta-hairpins that make up the smooth end of the beta-barrel, and irregular loops which make up the rough end of the beta-barrel. Located in the center of porins is a constriction zone located halfway down the water-filled channel called the eyelet. The eyelet contains both positively and negative charged amino acids. Positively charged amino acids consists of, three arginine, one histidine, and two lysine, which are located on one side of the beta-barrel wall. Negatively charged amino acids consist of, four glutamic acids and seven aspartic acids located on the opposite wall the beta-barrel. To counter act the excess of negative charge, there are two calcium ions attached near the eyelet. These positively and negatively charged amino acids are located at opposite sides of the water-filled channel to create an electric field. The inside of porins, the hole of the beta-barrel is composed of hydrophilic chains. Lastly, some porins have salt-bridges between their N-terminus and C-terminus. |
== Function == | == Function == | ||
Porins have many functions in context of its structural components. The main function of porins is passive diffusion channels so that sugars, ions, and amino acids may pass through the outer membrane. For this to occur however, several other functions from other components must occur. One of these components are the hydrophilic chains that line the inside of the beta-barrel of porins. The function of the hydrophilic chains is to create a polar environment within the ring to help sugars, ions, and amino acids to pass through. Another important component is the eyelet or constriction zone located inside the middle of the beta-barrel of porins. The eyelet blocks the water-filled channel of porins and determines what the size capacity and ion selectivity is for a solute to pass through. If a porin has salt-bridges between its N-terminus and C-terminus then it will create more stability for the structure. Lastly, the outside loops formed by the anti-parallel beta-sheets are rigid and packed close together. This allows porins to become highly resistant to proteases and detergents. | Porins have many functions in context of its structural components. The main function of porins is passive diffusion channels so that sugars, ions, and amino acids may pass through the outer membrane. For this to occur however, several other functions from other components must occur. One of these components are the hydrophilic chains that line the inside of the beta-barrel of porins. The function of the hydrophilic chains is to create a polar environment within the ring to help sugars, ions, and amino acids to pass through. Another important component is the eyelet or constriction zone located inside the middle of the beta-barrel of porins. The eyelet blocks the water-filled channel of porins and determines what the size capacity and ion selectivity is for a solute to pass through. If a porin has salt-bridges between its N-terminus and C-terminus then it will create more stability for the structure. Lastly, the outside loops formed by the anti-parallel beta-sheets are rigid and packed close together. This allows porins to become highly resistant to proteases and detergents. | ||
| Line 33: | Line 33: | ||
==PhoE:1PHO== | ==PhoE:1PHO== | ||
Unlike the 4gcp porin, some porins contain no ligands to operate as it's eyelet. An example of a porin without a ligand would be the 1pho<ref>Schirmer, T., Cowan, S.W., Jansonius, J.N. “1PHO.” RCSB PDB Protein Data Bank. 13 Jul. 2011. Web. 28 Nov. 2015. http://www.rcsb.org/pdb/explore/explore.do?structureId=1pho</ref> porin. [[Image:1pho.png | thumb | PhoE: 1pho]] Instead of having a ligand as it's eyelet, 1pho has a loop inside, which operates as it's constriction zone or eyelet. This loop is slightly anion selective and creates ion selectivity for molecules to pass through the porin. Other features of the 1pho porin is that they are found in E. coli, they are transports phosphate compounds, and they contain only one chain (A). The rest of the features of the 1pho porin are the same as the general structure of porins described earlier. | Unlike the 4gcp porin, some porins contain no ligands to operate as it's eyelet. An example of a porin without a ligand would be the 1pho<ref>Schirmer, T., Cowan, S.W., Jansonius, J.N. “1PHO.” RCSB PDB Protein Data Bank. 13 Jul. 2011. Web. 28 Nov. 2015. http://www.rcsb.org/pdb/explore/explore.do?structureId=1pho</ref> porin. [[Image:1pho.png | thumb | PhoE: 1pho]] Instead of having a ligand as it's eyelet, 1pho has a loop inside, which operates as it's constriction zone or eyelet. This loop is slightly anion selective and creates ion selectivity for molecules to pass through the porin. Other features of the 1pho porin is that they are found in E. coli, they are transports phosphate compounds, and they contain only one chain (A). The rest of the features of the 1pho porin are the same as the general structure of porins described earlier. | ||
| + | |||
Revision as of 01:27, 17 December 2015
This page is setup for Matt to build his senior project for OU CHEM 4923
|
Contents |
Porins Overview
Porins such as OmpC[1], are proteins located in the outer membrane of gram-negative bacteria or the outer membrane of mitochondria in eukaryotes and function as simple diffusion channels. The simple diffusion channels allow diffusion of sugars, ions, and amino acids to cross the outer membrane. Formation of porins occurs when a signal sequence is used to transfer the porin protein out from the inner membrane of bacteria. For this to occur, chaperone protein Sec B containing an unfolded polypeptide chain sends out a signal. This signal is sent to Sec A ATPase and binds to the Sec B complex. The polypeptide is then released and sent to the Sec E – Sec Y translocation channel where it begins to fold into partially assembled beta-sheets. The lipid-binding region located in the hydrophobic core of the partially assembled beta-sheets will then pull the complex into the outer membrane. Once in the outer membrane, the beta-sheets completely form by the alternating polar/non-polar side chains. Once porins have formed, structural components, functions, and energetic processes can be observed.
Structure
The general structure of porins is most recognized by its secondary structures.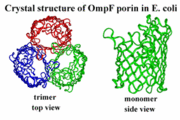
Function
Porins have many functions in context of its structural components. The main function of porins is passive diffusion channels so that sugars, ions, and amino acids may pass through the outer membrane. For this to occur however, several other functions from other components must occur. One of these components are the hydrophilic chains that line the inside of the beta-barrel of porins. The function of the hydrophilic chains is to create a polar environment within the ring to help sugars, ions, and amino acids to pass through. Another important component is the eyelet or constriction zone located inside the middle of the beta-barrel of porins. The eyelet blocks the water-filled channel of porins and determines what the size capacity and ion selectivity is for a solute to pass through. If a porin has salt-bridges between its N-terminus and C-terminus then it will create more stability for the structure. Lastly, the outside loops formed by the anti-parallel beta-sheets are rigid and packed close together. This allows porins to become highly resistant to proteases and detergents.
Energetics
The components and functions of porins can be brought together with its energetics to explain porins main function, simple diffusion.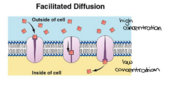
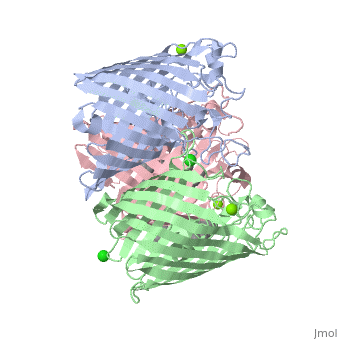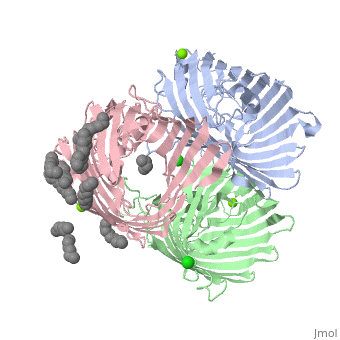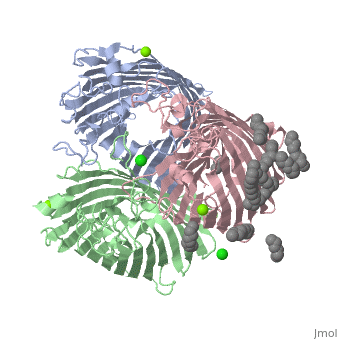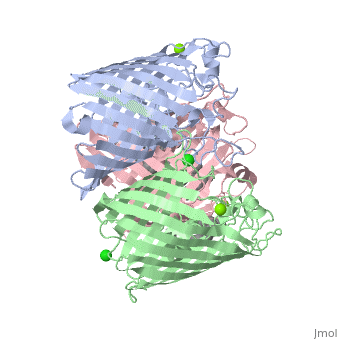
OmpF:4GCP
Certain porins such as 4gcp[6] contain ligands, which operate as it's eyelet. The ligand for 4gcp is an antibody, ampicillin, which is zwitterionic.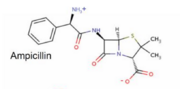
PhoE:1PHO
Unlike the 4gcp porin, some porins contain no ligands to operate as it's eyelet. An example of a porin without a ligand would be the 1pho[8] porin. Instead of having a ligand as it's eyelet, 1pho has a loop inside, which operates as it's constriction zone or eyelet. This loop is slightly anion selective and creates ion selectivity for molecules to pass through the porin. Other features of the 1pho porin is that they are found in E. coli, they are transports phosphate compounds, and they contain only one chain (A). The rest of the features of the 1pho porin are the same as the general structure of porins described earlier.
References
- ↑ http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15
- ↑ “Porin Channel Protein in Escherichia coli Outer Membrane.” Rs.Noda. Web. 4 Nov. 2015. http://www.rs.noda.tus.ac.jp/~biost/OPFU/yama/public_html/study/folding/md/porin/porin.htm
- ↑ “Biophysical Methods Lecture 4: Membrane Proteins – Porins.” Chembio. Department of Chemistry and Biochemistry University of Guelph. Nov. 2013. Web. 4 Nov. 2015. http://www.chembio.uoguelph.ca/educmat/phy456/456lec04.htm
- ↑ “What are the Different Modes of Biotransportation of Drugs – How Drugs Move Across the Cell Membrane?” HubPages. 28 Sept. 2015. Web. 4 Nov. 2015. http://hubpages.com/education/Different-modes-of-biotransport-of-drugs
- ↑ Casiday, Rachel. Frey, Regina. “Transport Across Membranes: Energetics and Pumps/Channels.” Academy of Gifted Learners. Department of Chemistry, Washington University. Web. 4 Nov. 2015. https://academyofgiftedlearners.files.wordpress.com/2014/11/chapter13_f141.pdf
- ↑ Ziervogel, B.K., Roux, B. “4GCP.” RCSB PDB Protein Data Bank. 6 Feb. 2013. Web. 28 Nov. 2015. http://www.rcsb.org/pdb/explore.do?structureId=4GCP
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3545085/figure/F1/ access 12/9/15
- ↑ Schirmer, T., Cowan, S.W., Jansonius, J.N. “1PHO.” RCSB PDB Protein Data Bank. 13 Jul. 2011. Web. 28 Nov. 2015. http://www.rcsb.org/pdb/explore/explore.do?structureId=1pho
[1]
Two in-text citations from same source:
First time it's referenced: [2]
Subsequent times: Cite error: Invalid <ref> tag;
refs with no name must have content