Sandbox myosinkinesin
From Proteopedia
| Line 15: | Line 15: | ||
== Structural highlights == | == Structural highlights == | ||
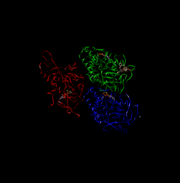
| + | [[Image:Hirokawa.jpg | Here is a picture of the light chains.]] | ||
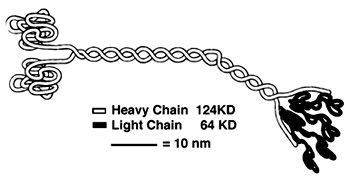
'''''Heavy Chain:''''' | '''''Heavy Chain:''''' | ||
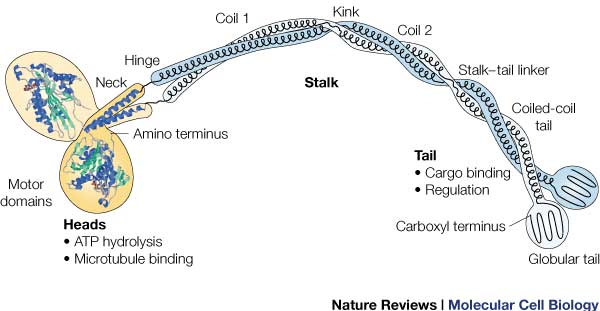
It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure. | It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure. | ||
| - | [[Image:Hirokawa.jpg | thumb | Here is a picture of the light chains.]] | ||
'''''Light Chain:''''' | '''''Light Chain:''''' | ||
Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. | Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. | ||
Revision as of 02:37, 17 December 2015
Kinesin
| |||||||||||
References
Goodsell, David. "Kinesin." RCSB PDB-101. Protein Data Bank, n.d. Web. 07 Nov. 2015.
Krukau, Aliaksei, Volker Knecht, and Reinhard Lipowsky. "Allosteric Control of Kinesin's Motor Domain by Tubulin: A Molecular Dynamics Study." Phys. Chem. Chem. Phys. Physical Chemistry Chemical Physics 16.13 (2014): 6189. Royal Society of Chemistry. Web. 6 Nov. 2015.
Kull, F. Jon, Elena P. Sablin, Rebecca Lau, Robert J. Fletterick, and Ronald D. Vale. "Crystal Structure of the Kinesin Motor Domain Reveals a Structural Similarity to Myosin." Nature 380.6574 (1996): 550-55. The National Center for Biotechnology Information. Web. 6 Nov. 2015.
Lodish, Harvey F., Arnold Berk, Paul Matsudaira, Chris A. Kaiser, Monty Krieger, Matthew P. Scott, Stephen Lawrence Zipursky, and James E. Darnell. Molecular Cell Biology. New York, NY: W.H. Freemann, 2003. Print.
Paxton, Walter F., Nathan F. Bouxsein, Ian M. Henderson, Andrew Gomez, and George D. Bachand. "Dynamic Assembly of Polymer Nanotube Networks via Kinesin Powered Microtubule Filaments." Nanoscale 7.25 (2015): 10998-1004. Royal Society of Chemistry. Web. 6 Nov. 2015.
Rayment, I., W. Rypniewski, K. Schmidt-Base, R. Smith, D. Tomchick, M. Benning, D. Winkelmann, G. Wesenberg, and H. Holden. "Three-dimensional Structure of Myosin Subfragment-1: A Molecular Motor." Science 261.5117 (1993): 50-58. PubMed. Web. 6 Nov. 2015.
Rice, Sarah, Abel W. Lin, Daniel Safer, Cynthia L. Hart, Nariman Naber, Bridget O. Carragher, Shane M. Cain, Elena Pechatnikova, Elizabeth M. Wilson-Kubalek, Michael Whittaker, Edward Pate, Roger Cooke, Edwin W. Taylor, Ronald A. Milligan, and Ronald D. Vale. "A Structural Change in the Kinesin Motor Protein That Drives Motility." Nature 402.6763 (1999): 778-84. Nature. Web. 6 Nov. 2015.
"SDSU Biology 590 - Actin Myosin Crossbridge 3D Animation." SDSU Biology 590 - Actin Myosin Crossbridge 3D Animation. San Diego State University College of Sciences, 30 Sept. 97. Web. 07 Nov. 2015.
Song, Y.-H. "Structure of a Fast Kinesin: Implications for ATPase Mechanism and Interactions with Microtubules." The EMBO Journal 20.22 (2001): 6213-225. PubMed. Web. 6 Nov. 2015.
https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html