Sandbox viralpackagingmotors
From Proteopedia
| Line 16: | Line 16: | ||
The P4 viral packaging motor is a sequential is a molecular machine that must complete several task in sequential order. After a successful packaging After successful packaging, the three ssRNAs are converted into dsRNA inside the viral procapsid by a virus-associated RNA-dependent RNA polymerase (protein P2). For each segment, the packaging process may be divided into three steps (Figure 1(D)): (i) recognition of the RNA pac site (a packaging signal) by a specific binding site on the surface of the viral capsid (i.e. major structural protein P1) [23,50], (ii) loading of the ssRNA into the packaging motor (most likely by a ring-opening mechanism) [43], (iii) unidirectional, 50–30 translocation of the captured ssRNA into the procapsid at the expense of ATP hydrolysis. The dome-shaped P4 hexamer has an overall structure and subunit fold similar to those of hexameric helicases (Figure 1(B)–(C)) [46,48]. The subunit structure has three domains: N-terminal apical dome, middle RecA-like ATPase core and C-terminal platform. Interestingly, the same basic fold was also found for a packaging ATPase domain from dsDNA bacteriophage T4. <ref name="RNA packaging motor"/> | The P4 viral packaging motor is a sequential is a molecular machine that must complete several task in sequential order. After a successful packaging After successful packaging, the three ssRNAs are converted into dsRNA inside the viral procapsid by a virus-associated RNA-dependent RNA polymerase (protein P2). For each segment, the packaging process may be divided into three steps (Figure 1(D)): (i) recognition of the RNA pac site (a packaging signal) by a specific binding site on the surface of the viral capsid (i.e. major structural protein P1) [23,50], (ii) loading of the ssRNA into the packaging motor (most likely by a ring-opening mechanism) [43], (iii) unidirectional, 50–30 translocation of the captured ssRNA into the procapsid at the expense of ATP hydrolysis. The dome-shaped P4 hexamer has an overall structure and subunit fold similar to those of hexameric helicases (Figure 1(B)–(C)) [46,48]. The subunit structure has three domains: N-terminal apical dome, middle RecA-like ATPase core and C-terminal platform. Interestingly, the same basic fold was also found for a packaging ATPase domain from dsDNA bacteriophage T4. <ref name="RNA packaging motor"/> | ||
<ref name= "ATPase that powers viral genome packaging">doi: 10.1073/pnas.1506951112</ref> <ref name="Atomic Snapshots"/>. | <ref name= "ATPase that powers viral genome packaging">doi: 10.1073/pnas.1506951112</ref> <ref name="Atomic Snapshots"/>. | ||
| - | Thus, based on structure alone one may be tempted to speculate that all these motor proteins may share certain mechanistic features. In recent years, the P4 protein has become one of the best characterized hexameric helicases and, given the wealth of structural and biophysical information available, it constitutes a good model system for studying the mechano-chemistry of nucleic acid translocation. <ref name="RNA packaging motor"/> | + | Thus, based on structure alone one may be tempted to speculate that all these motor proteins may share certain mechanistic features. In recent years, the P4 protein has become one of the best characterized hexameric helicases and, given the wealth of structural and biophysical information available, it constitutes a good model system for studying the mechano-chemistry of nucleic acid translocation. <ref name="RNA packaging motor"/> <ref name="Bacteriophage DNA Packaging Motor"> doi:10.1146/annurev.genet.42.110807.091545</ref>. |
== Function == | == Function == | ||
Revision as of 12:52, 17 December 2015
This page is setup for Ojuewa to build her senior project for OU CHEM 4923
Contents |
Viral Packaging
|
One of the most important stage in the life cycle of all viruses is the encapsidation (packaging) of the viral genome. Many virus package their genome into preformed capsids using packaging motors powered by the hydrolysis of ATP. The hexameric ATPase P4 of dsRNA bacteriophage phi 12, located at the vertices of the icosahedral capsid is such a packaging motor. [1] P4 protein is a 35-kDa ssRNA packaging ATPase from dsRNA bacteriophages belonging to the Cystoviridae family. ( phi 6 - phi 12). These viruses use a packaging motor like RNA packaging motor that is powered by the hydrolysis of ATP to condense the nucleic acid into a confined space.
Structure
The RNA packaging motor is a straightforward machinery that consists of a portal protein (P4) hexamer. The crystal structure of the hexameric P4 protein is made of three sub-units, one-half of the hexameric molecule (hexamers encirlce the crystallographic 2-fold axes). Residues 196-206, 300-306, and the C-terminal segment 322-331are disordered in all subunits.
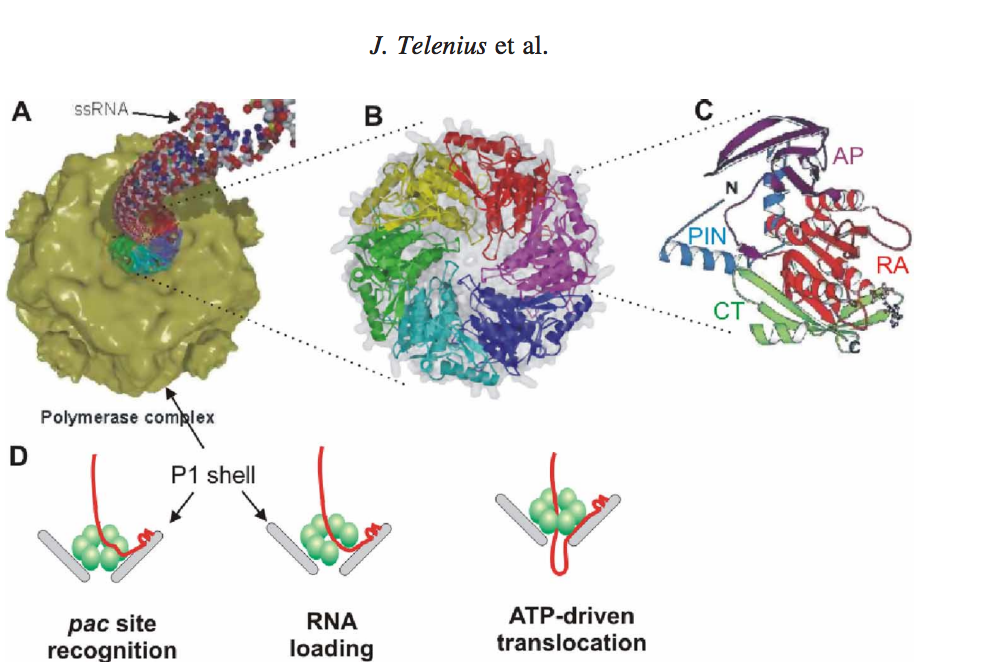 Figure 1. (A) Packaging of ssRNA via P4 hexamer into the icosahedral procapsid (B) P4 hexamer structure (C) Subunit structure, RecA-like catalytic core is shown in red (RA), N-terminal safety pin in cyan (PIN), apical domain in magenta (AP) and C-terminal platform in green (CT). The safety pin stabilizes interacts with a neighboring subunit and stabilizes the hexamer. (D) A scheme of the three step packaging mechanism for a ssRNA precursor.
[2]
The P4 viral packaging motor is a sequential is a molecular machine that must complete several task in sequential order. After a successful packaging After successful packaging, the three ssRNAs are converted into dsRNA inside the viral procapsid by a virus-associated RNA-dependent RNA polymerase (protein P2). For each segment, the packaging process may be divided into three steps (Figure 1(D)): (i) recognition of the RNA pac site (a packaging signal) by a specific binding site on the surface of the viral capsid (i.e. major structural protein P1) [23,50], (ii) loading of the ssRNA into the packaging motor (most likely by a ring-opening mechanism) [43], (iii) unidirectional, 50–30 translocation of the captured ssRNA into the procapsid at the expense of ATP hydrolysis. The dome-shaped P4 hexamer has an overall structure and subunit fold similar to those of hexameric helicases (Figure 1(B)–(C)) [46,48]. The subunit structure has three domains: N-terminal apical dome, middle RecA-like ATPase core and C-terminal platform. Interestingly, the same basic fold was also found for a packaging ATPase domain from dsDNA bacteriophage T4. [2]
[3] [1].
Thus, based on structure alone one may be tempted to speculate that all these motor proteins may share certain mechanistic features. In recent years, the P4 protein has become one of the best characterized hexameric helicases and, given the wealth of structural and biophysical information available, it constitutes a good model system for studying the mechano-chemistry of nucleic acid translocation. [2] [4].
Figure 1. (A) Packaging of ssRNA via P4 hexamer into the icosahedral procapsid (B) P4 hexamer structure (C) Subunit structure, RecA-like catalytic core is shown in red (RA), N-terminal safety pin in cyan (PIN), apical domain in magenta (AP) and C-terminal platform in green (CT). The safety pin stabilizes interacts with a neighboring subunit and stabilizes the hexamer. (D) A scheme of the three step packaging mechanism for a ssRNA precursor.
[2]
The P4 viral packaging motor is a sequential is a molecular machine that must complete several task in sequential order. After a successful packaging After successful packaging, the three ssRNAs are converted into dsRNA inside the viral procapsid by a virus-associated RNA-dependent RNA polymerase (protein P2). For each segment, the packaging process may be divided into three steps (Figure 1(D)): (i) recognition of the RNA pac site (a packaging signal) by a specific binding site on the surface of the viral capsid (i.e. major structural protein P1) [23,50], (ii) loading of the ssRNA into the packaging motor (most likely by a ring-opening mechanism) [43], (iii) unidirectional, 50–30 translocation of the captured ssRNA into the procapsid at the expense of ATP hydrolysis. The dome-shaped P4 hexamer has an overall structure and subunit fold similar to those of hexameric helicases (Figure 1(B)–(C)) [46,48]. The subunit structure has three domains: N-terminal apical dome, middle RecA-like ATPase core and C-terminal platform. Interestingly, the same basic fold was also found for a packaging ATPase domain from dsDNA bacteriophage T4. [2]
[3] [1].
Thus, based on structure alone one may be tempted to speculate that all these motor proteins may share certain mechanistic features. In recent years, the P4 protein has become one of the best characterized hexameric helicases and, given the wealth of structural and biophysical information available, it constitutes a good model system for studying the mechano-chemistry of nucleic acid translocation. [2] [4].
Function
The RNA packaging motor is a straightforward machinery that consists of a portal protein (P4) hexamer.
Energetics
The P4 protein provides energy for the RNA translocation.
Structural highlights
The structurally conserved Rec-A like core encompasses five sequence motifs (H1, H1a, H2, H3 and H4) that are common to all superfamily 4 (SF4) helicases (Figure 1(C)). The structural homology of P4 proteins extends to SF3 (human papilloma virus HPV E1, Simian virus 40 SV40 Large T antigen and LTag) and SF5 (Rho terminator) helicases, and to other proteins of the AAA+ family. [2] It is important to note that the sensor motifs that are supposed to detect the presence or absence of γ-phosphate of ATP and relay the information to other sites within the molecule through conformational changes were first identified as RecA. The active sites of RecA like ATPases usually contain a second motif contributed from an adjacent monomer, commonly an Arginine residue, containing the γ-phosphate of ATP. [2] [3]. φ12 p4 appears to have two arginine fingers residue Arg 272 and Arg 279 located on either side of the a β hairpin. Insertion of this "arginine finger" into the catalytic site is believed to stabilize the transition state, thus facilitating ATP-hydrolysis. These arginines are connected via strand β15 to Try 288, which forms part of an adjacent nucleotide binding site providing a mechanism to transit conformational changes between neighboring active sites upon nucleotide binding and hydrolysis [2] [1].
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ 1.0 1.1 1.2 Mancini EJ, Kainov DE, Grimes JM, Tuma R, Bamford DH, Stuart DI. Atomic snapshots of an RNA packaging motor reveal conformational changes linking ATP hydrolysis to RNA translocation. Cell. 2004 Sep 17;118(6):743-55. PMID:15369673 doi:10.1016/j.cell.2004.09.007
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 http://dx.doi.org/10.1080/17486700802168502
- ↑ Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647-81. doi: 10.1146/annurev.genet.42.110807.091545. PMID:18687036 doi:http://dx.doi.org/10.1146/annurev.genet.42.110807.091545
Jelena Telenius, Anders E. Wallin, Michal Straka, Hongbo Zhang, Erika J. Mancini and Roman Tuma. RNA packaging motor: from structure to quantum mechanical modelling and sequential-stochastic mechanism. Computational and Mathematical Methods in Medicine Vol. 9, Nos. 3-4, September -December 2008, 351-369 [1]