Sandbox myosinkinesin
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
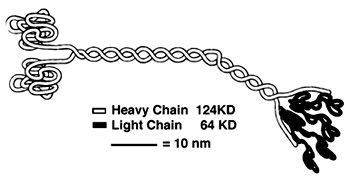
'''''Heavy Chain:''''' | '''''Heavy Chain:''''' | ||
| - | It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure. | + | It is the most conserved region amongst kinesin which consists of the head, neck, and tail. Usually contains eight core β-sheets and six major alpha helixes, most of these secondary structures are in different places in the primary sequence but line up in the tertiary structure.<ref name= "OP"/> |
'''''Light Chain:''''' | '''''Light Chain:''''' | ||
Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. Is usually alpha helical in structure. <ref> PMID:8316857</ref> | Not technically part of the protein of kinesin or myosin itself, but its presence is necessary for activity. It regulates conformational changes within the protein. Is usually alpha helical in structure. <ref> PMID:8316857</ref> | ||
| Line 29: | Line 29: | ||
ATPase: It generates this force using an ATPase either coupled with the myosin/kinesin or inherent within the head, the inherent ATPase uses a P-loop, which is a phosphate- binding loop used in adenylate kinase, Ras, and others. Both also create a similar environment for the γ-phosphate, which uses a conserve motif of Ser-Ser-Arg.<ref name= "RAS">doi: 10.1038/380550a0</ref> | ATPase: It generates this force using an ATPase either coupled with the myosin/kinesin or inherent within the head, the inherent ATPase uses a P-loop, which is a phosphate- binding loop used in adenylate kinase, Ras, and others. Both also create a similar environment for the γ-phosphate, which uses a conserve motif of Ser-Ser-Arg.<ref name= "RAS">doi: 10.1038/380550a0</ref> | ||
| - | In kinesin, the binding of ATP greatly increases the binding of kinesin to the microtubules, to allow it to perform a power stroke. | + | In kinesin, the binding of ATP greatly increases the binding of kinesin to the microtubules, to allow it to perform a power stroke.<ref name= "OP"/> |
| - | RAS fold: conserved domain among many signaling proteins. A structural motif where a nucleotide molecule is bound to loops at one end of a β-sheet domain. This suggests that myosin and kinesin may have evolved from a common ancestor.<ref name= "RAS"/> | + | ''RAS fold'': conserved domain among many signaling proteins. A structural motif where a nucleotide molecule is bound to loops at one end of a β-sheet domain. This suggests that myosin and kinesin may have evolved from a common ancestor.<ref name= "RAS"/> |
| Line 40: | Line 40: | ||
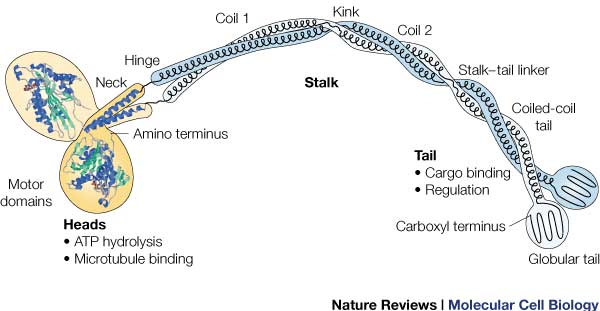
'''Tail:''' | '''Tail:''' | ||
| - | Includes polypeptide binding site, varies the most since it is responsible for dictating the location within the cell that the protein is active. | + | Includes polypeptide binding site, varies the most since it is responsible for dictating the location within the cell that the protein is active. <ref name= "OP"/> |
</StructureSection> | </StructureSection> | ||
Revision as of 18:24, 17 December 2015
Kinesin
| |||||||||||
References
- ↑ doi: https://dx.doi.org/10.2210/rcsb_pdb/mom_2005_4
- ↑ Hackney, David. "Kinesin-1 Structure." Duke. Duke, 25 Jan. 2005. Web. 17 Dec. 2015. <https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html>.
- ↑ 3.0 3.1 3.2 3.3 Song YH, Marx A, Muller J, Woehlke G, Schliwa M, Krebs A, Hoenger A, Mandelkow E. Structure of a fast kinesin: implications for ATPase mechanism and interactions with microtubules. EMBO J. 2001 Nov 15;20(22):6213-25. PMID:11707393 doi:10.1093/emboj/20.22.6213
- ↑ Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857
- ↑ Krukau A, Knecht V, Lipowsky R. Allosteric control of kinesin's motor domain by tubulin: a molecular dynamics study. Phys Chem Chem Phys. 2014 Apr 7;16(13):6189-98. doi: 10.1039/c3cp53367k. PMID:24561904 doi:http://dx.doi.org/10.1039/c3cp53367k
- ↑ 6.0 6.1 Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996 Apr 11;380(6574):550-5. PMID:8606779 doi:http://dx.doi.org/10.1038/380550a0
- ↑ 7.0 7.1 Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD. A structural change in the kinesin motor protein that drives motility. Nature. 1999 Dec 16;402(6763):778-84. PMID:10617199 doi:http://dx.doi.org/10.1038/45483